Most of us are familiar with anticoagulant rodenticide toxicosis and the range of clinical signs it can present with, but there is one potentially fatal manifestation of coagulation pathology that is perhaps not as widely known…
Dogs with severe clotting problems will occasionally bleed into the dorsal tracheal membrane. This causes collapse of the thoracic trachea and can lead to severe respiratory distress.
Presenting signs
These cases can present with none of the other signs of bleeding normally associated with coagulopathies, so rat bait poisoning may not come to mind as a differential diagnosis if you are not aware of this syndrome.
The typical case will present as an otherwise healthy dog that develops acute respiratory problems. Early signs can be as mild as a persistent cough, but it can quickly escalate into a life-threatening respiratory crisis.
Severe cases will have an obvious stridor on both inspiration and expiration, cyanotic mucous membranes, and patients may be very distressed.
It will look very much like:
- a dog that is choking from a tracheal foreign body
- an old dog with tracheal collapse
- the end stages of laryngeal paralysis – except the stridor will come from much lower in the respiratory tract than it does in laryngeal paralysis
So, what do you do?
On initial presentation you would approach it as any respiratory distress case: oxygen, oxygen, oxygen, calm and stress-free handling, and light sedation (butorphanol, for example).
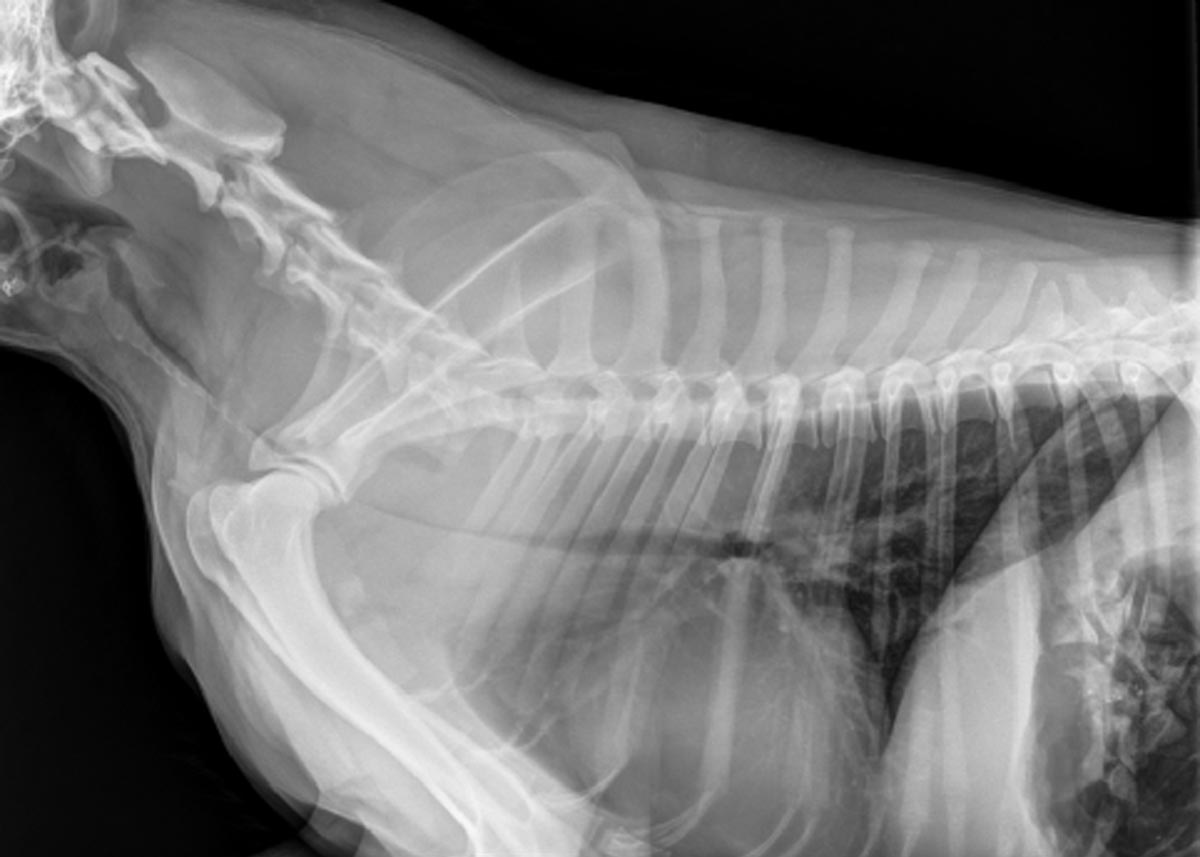
Once it is safe to do so, you should take chest rads to look for what you’ll probably suspect is a tracheal foreign body, and you’ll get an image like the one above (although it may not be this severe). Then you’ll remember this article, have an “aha!” moment and run a clotting profile (but if it’s as bad as this case, you’ll obviously first save the animal’s life by passing an ET tube).
Once a clotting problem is confirmed you’ll need to stop the bleeding with standard therapy for anticoagulant rodenticide toxicity: plasma and vitamin K.
Severe cases
In a severe case you may need to keep the dog intubated for several hours, until the clotting times have normalised, before cautiously attempting to extubate.
If the patient is unable to stay well oxygenated without an ET tube (mucous membrane colour, pulse oximetry, blood gas), consider placing a long oxygen catheter past the narrowing – either via a tracheostomy or a nasal O2 catheter.
If these cases are quickly recognised for what they are, and an open airway can be maintained, the prognosis should be good. These are potentially very satisfying cases with great potential for you to be a total hero.