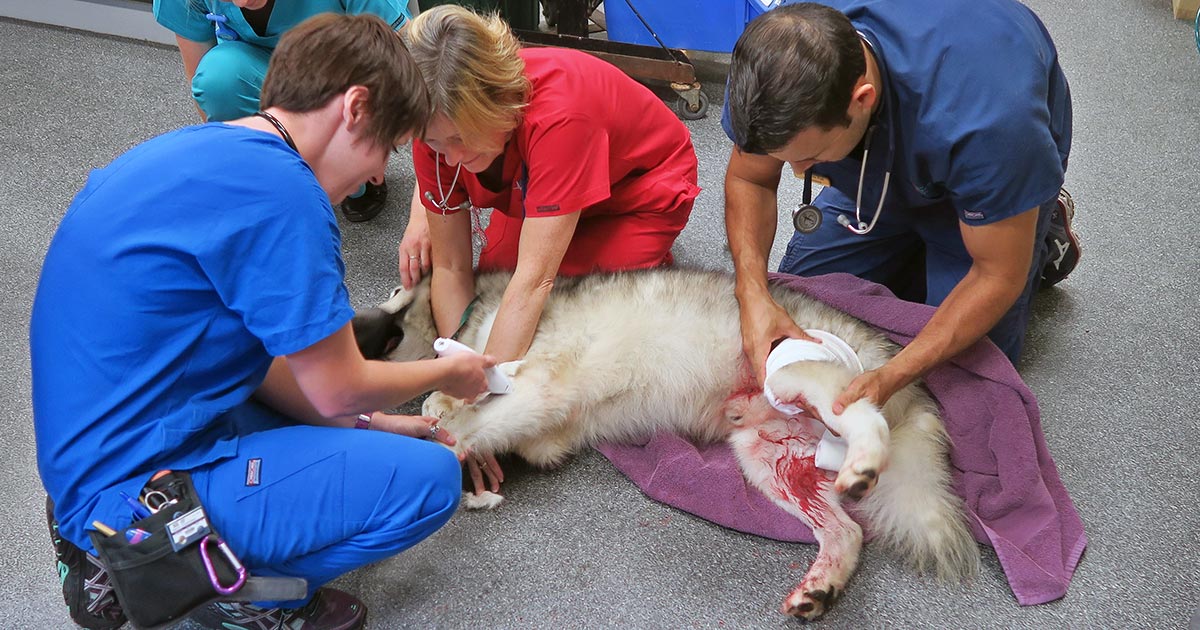
The art of triage takes time to master – particularly in emergency hospitals, where critical patients arrive in quick succession to the crash area.
Patients need to be examined quickly and effectively to ensure the most critical issues are identified and stabilised. To do this, I break triage into two categories – primary survey and secondary survey.
In part one I will discuss the primary survey process. The second part will go into the details of the secondary survey.
ABCDE approach
Primary survey refers to the initial stabilisation stage, where the aim is to preserve life, manage life-threatening injuries and re-establish tissue perfusion with oxygenated blood.
The approach to all deteriorating or critical patients is the same: ABCDE – Airway, Breathing, Circulation, Disability and External.
ABC – Airway, Breathing, Circulation

Assess airway
- Is it patent?
- Does it require suctioning?
- Does evidence exist of upper airway obstruction?
- Intubate if you suspect the patient may need resuscitation.
If you suspect cardiopulmonary arrest, begin basic life support immediately. This involves chest compressions, and intubation and ventilation in:
- loss of consciousness
- absence of spontaneous ventilation
- absence of heart sounds on auscultation
- absence of palpable pulses
Assess breathing
- Is the patient hyperventilating or hypoventilating?
- How is the respiratory effort? Is it sustainable?
- What is its oxygen saturation, or does the patient looks like it needs oxygen? If yes, provide supplemental oxygen therapy.
- Does the patient need to be ventilated? Ventilation is required if any of these criteria are satisfied:
- hypoxaemia (partial pressure of oxygen lower than 60mmHg or blood oxygen saturation level lower than 90%) and unresponsive to oxygen supplementation
- hypercapnia or hypoventilation (partial pressure of carbon dioxiden greater than 60mmHg)
- unsustainable respiratory effort?
- Consider sedation. Reducing stress can make a dramatic difference in stabilising dyspnoeic patients. Butorphanol or low-dose acepromazine (try to rule out cardiac disease first) can be used in these situations.
Assess cardiovascular system
- Mucous membrane colour, capillary refill time, heart rate and rhythm, pulse pressure, and temperature.
- If a heart murmur or arrhythmia is present, I try to rule out cardiogenic shock before resuscitation therapy.
At the same time, draw enough blood to run baseline blood work and begin IV fluid resuscitation if signs of shock are present. Fluid boluses should be considered if any of these exist:
- pale mucous membranes
- slow capillary refill time
- tachycardia
- poor pulse pressures
- hypothermia
I generally start with 10ml/kg Hartmann’s Solution over five minutes and reassess.
D – Disability assessment
Any abnormalities in:
- Mentation? Seizures?
- Level of consciousness?
- Altered pain sensation?
E – External assessment
- assessment for wounds or injuries
- control any obvious bleeding, apply direct pressure – possibly tourniquets, but only for less than 30 minutes unless life-threatening bleeding
- initial medications:
- pain relief – generally opioids are safest with unstable patients
- antiepileptics – diazepam
- sedation for dyspnoeic patients – butorphanol or low-dose acepromazine IV
Once you have finished your primary survey and performed the required intervention, you repeat your primary survey until the patient is appropriately stabilised.