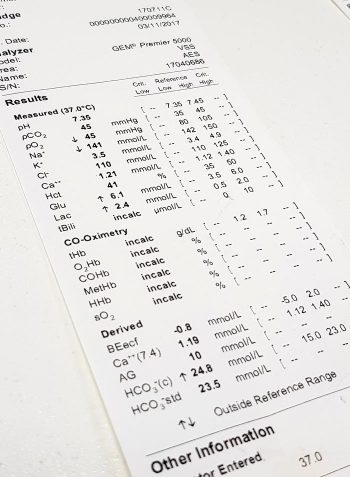
Simple acid-base disorders are compensated by predictable compensatory changes. The primary disorder shifts the pH, while the compensatory mechanisms aim to normalise the pH and bring it back to neutral.
This is achieved by attempting to normalise the bicarbonate (HCO3-) to partial pressure of CO2 (PCO2) ratio in a paralleled manner.
For example, an increase in HCO3– (metabolic alkalosis) is compensated by an increase in PCO2 (respiratory acidosis). Similarly, a respiratory alkalosis (decrease in PCO2) is compensated by a metabolic acidosis (decrease in HCO3-).
Ruling out secondary process
However, before jumping to the conclusion an opposing change is the result of compensation, we must rule out the presence of a secondary process. This can only be determined by calculation (Table 1).
| Table 1. Calculating compensatory change | |
|---|---|
| Component | Expected compensation |
| Metabolic acidosis ↓HCO3 (↓BE) |
per 1mEq/L ↓ in HCO3 = ↓ PCO2 of 0.7mmHg |
| Metabolic alkalosis ↑HCO3 (↑BE) |
per 1mEq/L ↑ in HCO3 = ↑ PCO2 of 0.7mmHg |
| Respiratory acidosis (acute) ↑PCO2 |
per 1mmHg ↑ PCO2 = ↑ 0.15mEq/L HCO3 |
| Respiratory acidosis (chronic) ↑PCO2 |
per 1mmHg ↑ PCO2 = ↑ 0.35mEq/L HCO3 |
| Respiratory alkalosis (acute) ↓PCO2 |
per 1mmHg ↓ PCO2 = ↓ 0.25mEq/L HCO3 |
| Respiratory alkalosis (chronic) ↓PCO2 |
per 1mmHg ↓ PCO2 = ↓ 0.55mEq/L HCO3 |
By comparing the reported to what the calculated compensatory change should be, you can determine whether the patient’s reported value is due to compensation or a separate disorder – for example, multiple primary acid-base disorders (a mixed acid-base disorder).
An example of a mixed disturbance could be a hyperventilating (respiratory alkalosis) dog with renal failure (metabolic acidosis).
The level of decrease in PCO2 change is in excess of the calculated compensation for the metabolic acidosis, therefore confirming a mixed acid-base disturbance. In fact, the most common causes of hyperventilation – pain, fear and excitement – often complicate blood gas analysis.
Another example of a mixed disorder could be a patient with traumatic haemothorax experiencing both lactic acidosis (hypoperfusion) and hypoventilation (respiratory acidosis) due to pleural space disease.
Waiting game
Another thing to keep in mind is compensation takes time – respiratory processes take approximately 8 to 12 hours, while metabolic processes take one to three days.
The lungs are able to alter PCO2 levels relatively quickly by adjusting the rate of ventilation. The kidneys, on the other hand, take a longer time to adjust the pH, as the change in rate of absorption and excretion of HCO3– takes much longer in comparison.
Regardless of the rate, physiologic compensation for a primary acid-base disturbance is almost never able to return pH to neutral.
Summary
A simple acid-base disorder should be suspected when the patient’s reported values are similar to the calculated compensation value, and a mixed acid-base disorder when the values fall outside the calculated range.
Another hint that a mixed acid-base disturbance is present is if the pH falls within the normal reference range, but the HCO3– or the PCO2 are not; or if the HCO3– and PCO2 are in opposite directions as opposed to being parallel.
Remember, the body can never overcompensate nor return the pH to neutral.



 For cases with a normal pH, we need to determine which category it falls into:
For cases with a normal pH, we need to determine which category it falls into: Once the primary disorder has been identified, we need to look at whether a secondary disorder is also present and, if so, whether this is the result of compensation or a true mixed process.
Once the primary disorder has been identified, we need to look at whether a secondary disorder is also present and, if so, whether this is the result of compensation or a true mixed process.