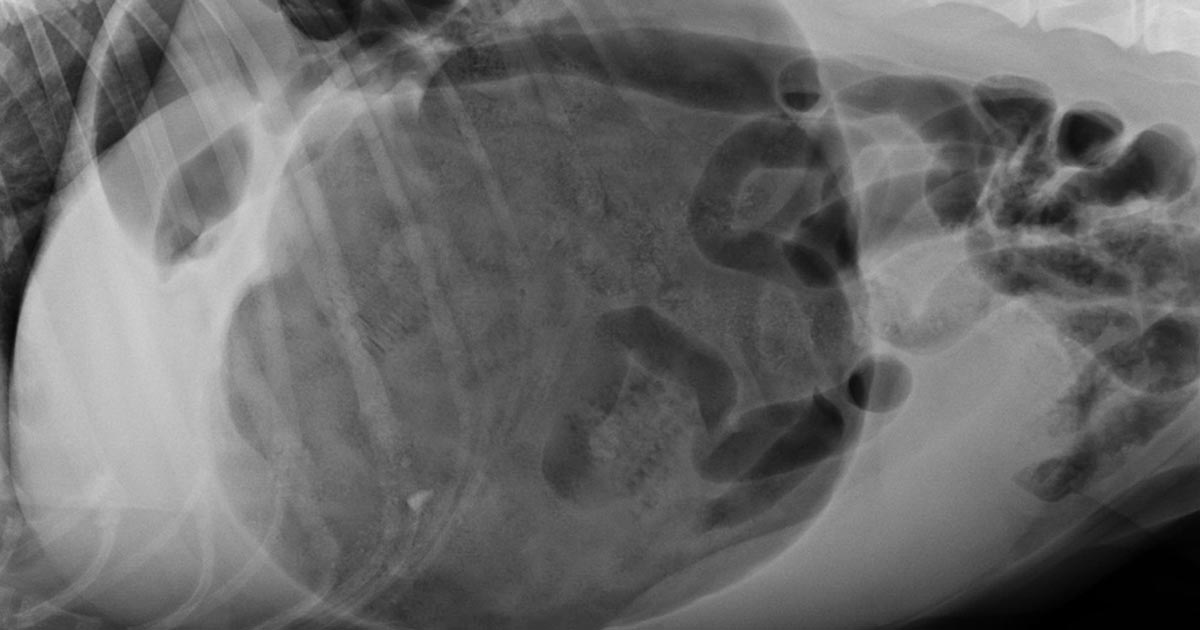
You’re stuck in the clinic in the middle of the night with a dog that is dying – it’s bleeding into its abdomen and needs blood, but the bag in the fridge is expired.
You’ve heard it’s possible to collect the blood out of the abdomen and safely give it to the patient, but you’re not sure how and don’t have time to google. How do you actually do it?
It’s easier than you think. Depending on what equipment you have available in the clinic, here are two practical ways of administering an autotransfusion.
Collection
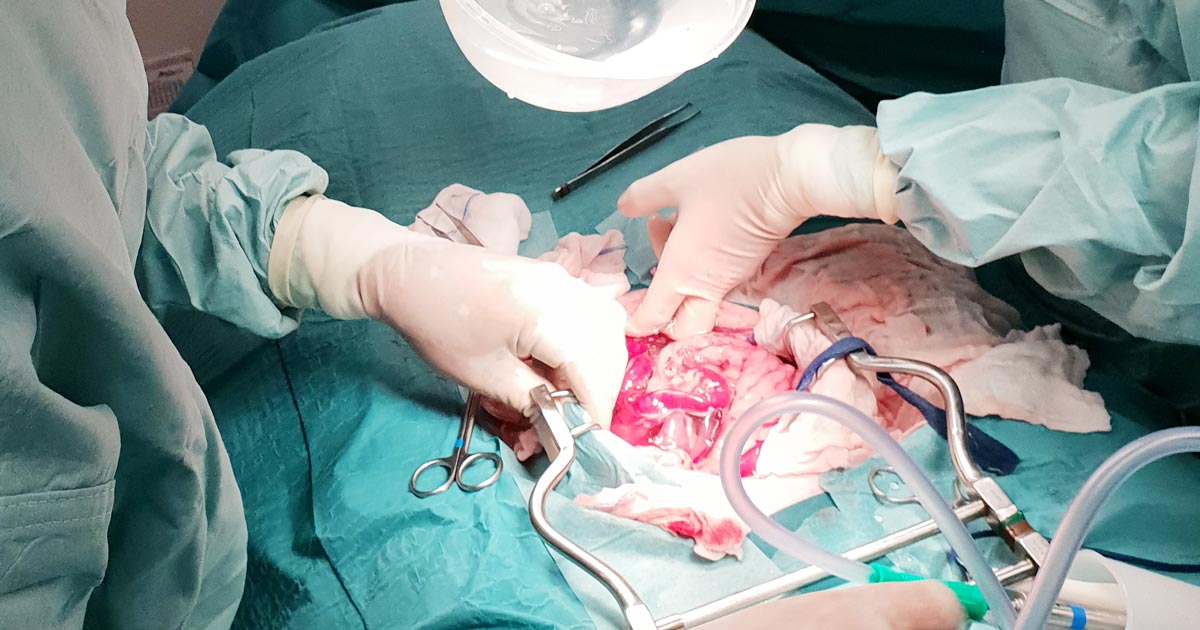
Blood can be collected from the abdomen or thorax during exploratory surgery, simply by sucking it up with a 60ml syringe.
Time is usually of the essence in these cases, so a handy tip is to use 60ml catheter tip syringes to aspirate the blood, then get someone to transfer the blood into a standard Luer-tipped syringe by shoving the smaller tip into the catheter tip. Like this:
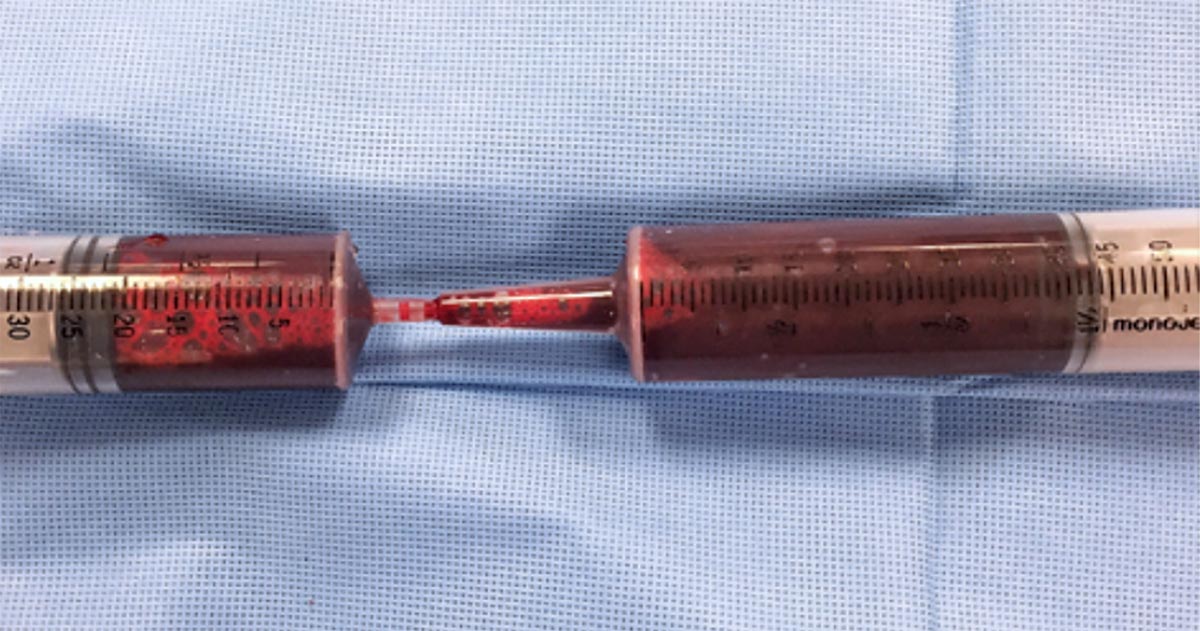
Besides the faster collection time, the bigger aperture of the catheter tip syringe will also reduce negative pressure during collection and, therefore, the chances of haemolysis.
If you are not going into surgery, you can collect the blood percutaneously – using a butterfly needle or catheter – as you would drain any body cavity fluid.
You shouldn’t need to add any anticoagulants to your syringes, as fibrinolysis has already occurred – meaning the blood shouldn’t clot in your syringe.
Administration
Whichever method you use, it’s crucial the blood is administered through a blood filter.
Method one
The most straightforward way is to simply inject the blood back into the patient using the Luer-tipped syringe through a filter. For this technique, you will need a filter that can connect to a syringe.
Method two
If you only have a blood filter as part of a blood giving set, you could use a two-way stopcock valve to get your collected blood into a sterile bag – like an empty IV fluids bag, or a dedicated blood collection bag – and then run the blood through your blood giving set with the built-in filter, as you would for a normal transfusion.
This is a bit more fiddly, so adding a few little blood filters to your emergency equipment could be well worth your time.
When you shouldn’t do it
Autotransfusions are most useful in cases of bleeding due to clotting deficiencies where the clotting problem is already being addressed, but a need for red blood cells still exists; and for cases of traumatic or postoperative bleeding.
It is contraindicated if contamination with urine, faeces, bile or bacteria – in case of septic peritonitis – is likely; or in cases of neoplasia in the body cavity where an autotransfusion has the potential to seed neoplastic cells to the rest of the body.

 Numerous online calculators can determine whether a toxic dose has been consumed and they are a great place to start.
Numerous online calculators can determine whether a toxic dose has been consumed and they are a great place to start.