Few companies now offer affordable point-of-care tests for canine C-reactive protein (CRP). As we did when we recently received our new box of CRP slides, you might soon be asking the question: what do we even do with this stuff?
Here’s what we’ve learnt…
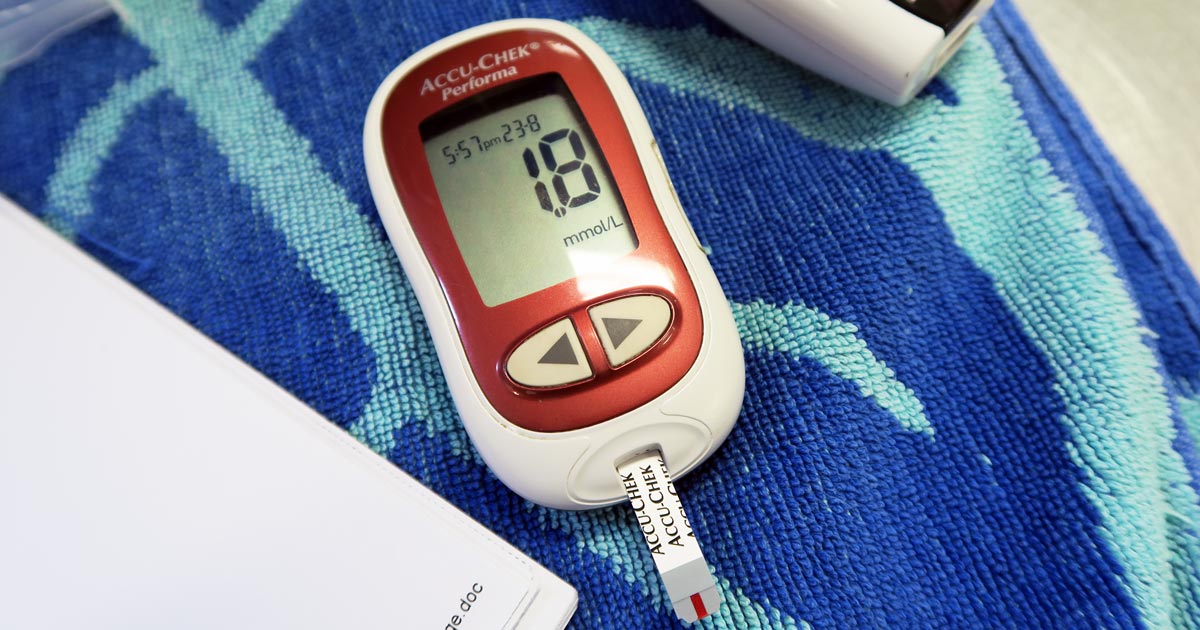
CRP is one of the acute phase proteins produced by the liver in response to inflammation. Healthy patients have very low levels of CRP, but a systemic inflammatory condition will cause an increase in CRP within four to six hours. Conversely, increased levels will decrease rapidly on resolution of inflammation. This provides an almost real time measure of inflammation that is more responsive and reliable than the white blood cell response.
In other words, CRP can indicate the presence of inflammation before the patient’s white blood cell count gives any clues, or before it becomes pyrexic – and, unlike the white blood cell count, stress and steroids do not affect CRP levels.
Uses
So, how do we use it?
- I love it for early pickups of problems in those grey area cases: the dog seems okay on clinical examination, but something about it bothers me. A normal or mildly increased CRP test will make me sleep more easy, while a surprise high reading will prompt me to admit for full diagnostics, or at least get the patient in for a follow-up CRP the next day. Conversely, a localised problem – such as an abscess – combined with a normal CRP test might mean you can hold off on antibiotics and just recheck CRP in 24 hours.
- It’s great for monitoring response to treatment. If my plan is working then I’d expect CRP to show a significant decrease by day two or three. If it’s not dipping by then, I need to reassess my treatment plan. Do I need to change antibiotics? Scan it again? Maybe we need to consider surgery? It can also be a good prognosticator. Research has shown failure of CRP to decrease significantly (around a 3× decrease) by around day three is generally bad news for patients with inflammatory conditions such as pancreatitis and immune-mediated haemolytic anaemia.
- We are starting to play with it for post-surgical monitoring. Any surgery will cause inflammation with an increase in CRP levels, but in an uncomplicated postoperative period, you should expect levels to start decreasing by day three to five. A base line CRP 24 hours after surgery with a recheck on day three should pick up early signs of postoperative problems such as infection, and prompt investigation or intervention.
- A potentially nifty use for it that we haven’t yet had the opportunity to use is in differentiating inflammatory lamenesses (arthritis, infection, injury) from a neurological causes – that is, is it arthritis or a nerve problem?
Limitations
- Remember, it’s very sensitive, so will increase with almost any inflammation. A mild upper respiratory infection or a bad gingivitis will likely induce some changes, so it’s important not to over-interpret (keep in mind that the magnitude of the increase in CRP does generally correspond with the severity of the inflammatory response). A pancreatitis case where the CRP fails to drop does not always mean death is looming – you may have just missed the concurrent skin disease. Always interpret CRP values in concert with your clinical examination.
- Be aware that pregnancy and intense exercise can increase CRP values.
- Not all serious conditions have an inflammatory component. CRP will be unchanged in most veterinary cases of heart disease; in common hormonal disease, such as adrenal disease and uncomplicated diabetes; urinary obstructions; many localised cancers; epilepsy and many others. Don’t presume that just because CRP is normal, everything is fine.
- No similar test exists for cats.
Sit up and say…
My favourite way to explain how to use this test is by using its highly appropriate acronym – any unexpected increase should make you sit up and say: “Oh CR*P! What am I missing?”