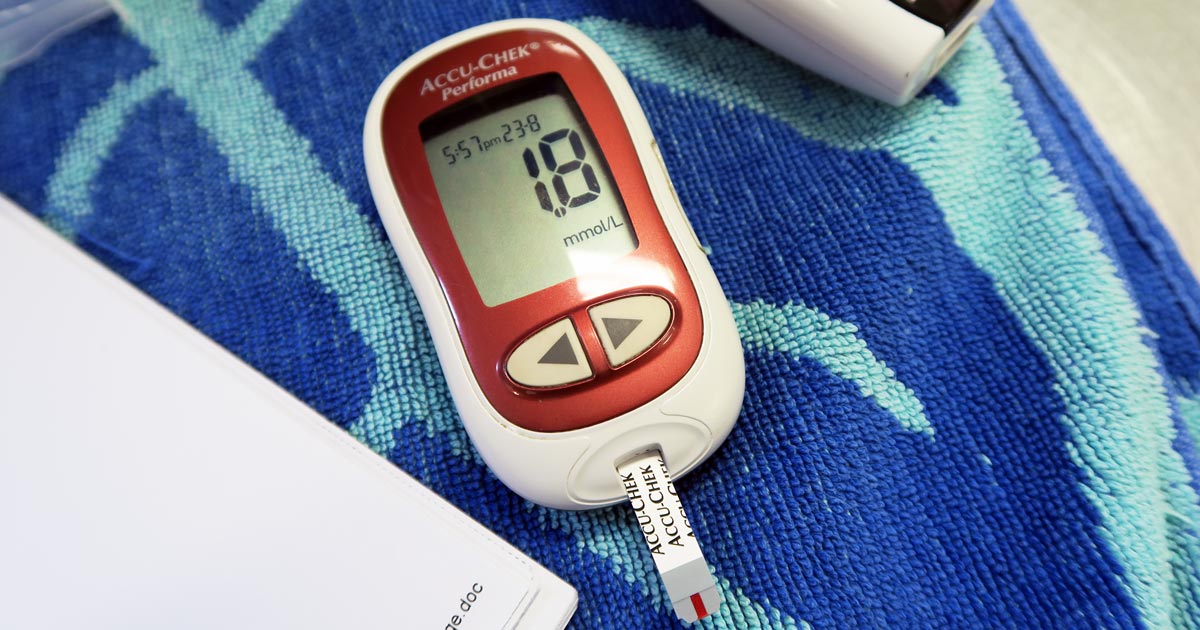
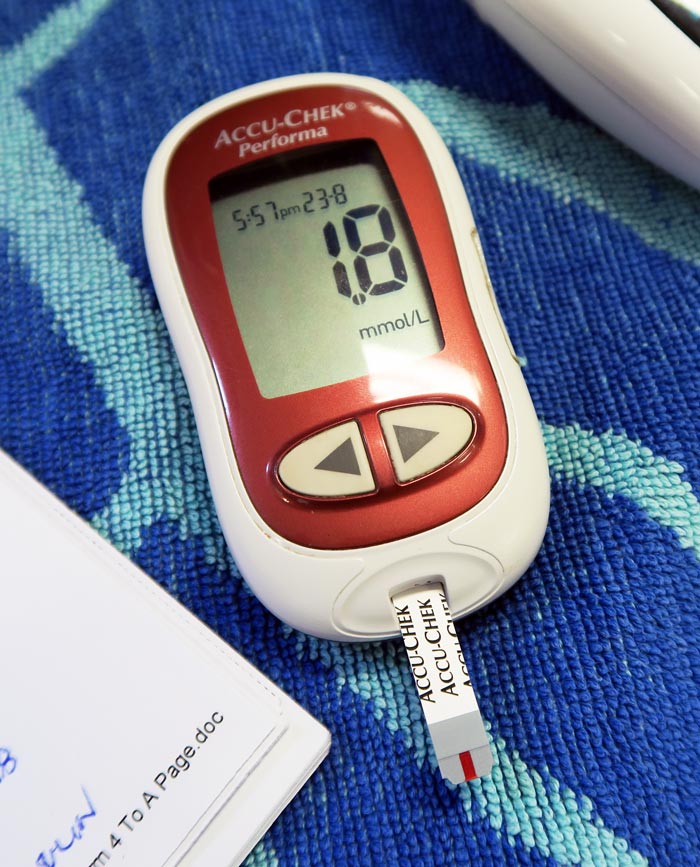
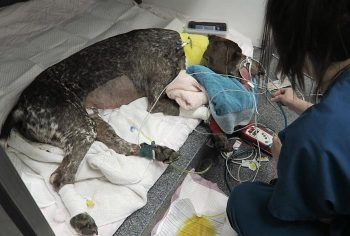
Several easy and affordable ways exist to measure lactate in general practice, which means the clinical applications of monitoring lactate is no longer the reserve of specialist and emergency centres.
But why and how should you be using it in general practice?
What is lactate again?
When oxygen is not effectively delivered to cells throughout the body – which, in our patients, will mostly be due to hypoperfusion (for example, hypovolaemia, vasodilatory shock and cardiac disease) – cells will switch from aerobic to anaerobic metabolism to stay alive.
Think of anaerobic metabolism as the fuel-powered generator that kicks in during a power cut – it’s not as good, but it’ll keep the lights on for a while. However, unless the power comes back on, the generator will eventually also fail and plunge you into darkness.
Lactate is the end product of this process of anaerobic metabolism. To be clear, lactate is not the bad guy – in fact, it plays an important role in keeping the cells going until they have access to sufficient oxygen again. It’s simply the bearer of bad news.
Why should I care?

Because lactate is the harbinger of doom; it’s the leading horseman of the apocalypse…
When lactate is high, you should stop whatever else you are doing and pay attention to the patient in question – this patient is probably surviving on anaerobic metabolism. It is critically ill and possibly heading for a long walk in the great park in the sky…
How should I use it?
It is valuable in any patient with any serious illness or injury. Things that should make you consider checking lactate would be:
- slow capillary refill time
- any mucous membrane colour other than a nice, healthy pink
- increased heart rate
- weak or bounding pulses
- significant dehydration
- depressed mentation
- history of major trauma
- major infections
- significant blood loss
- any disease that has the potential to progress into a life-threatening condition
…basically, any animal sick enough to make you worry about it possibly dying.
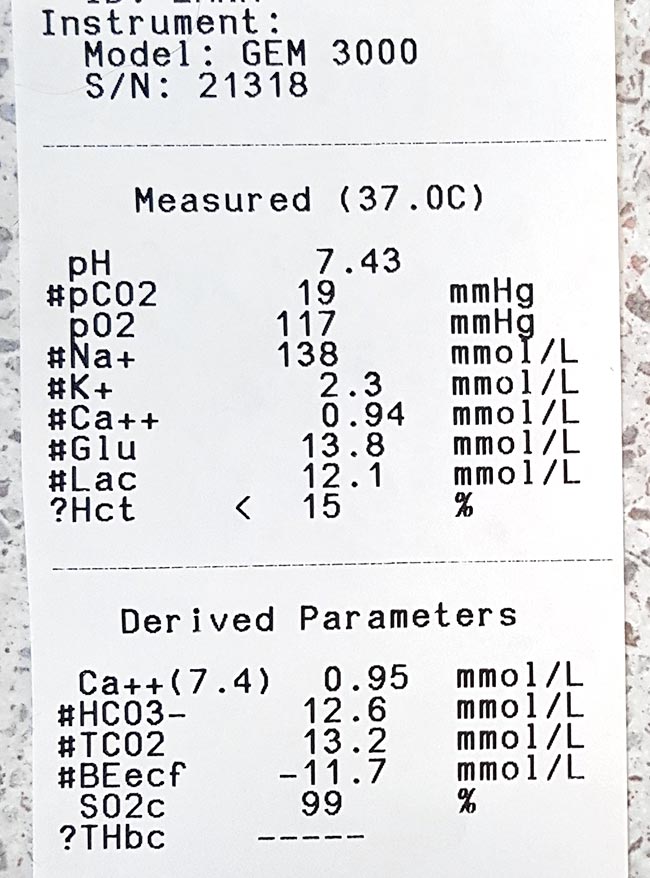
Run it with your initial diagnostics to get a baseline level, and run it within 10 minutes of taking the sample.
What do I do about my results?
Normal range
< 2.5 ?
3-4 ? ?
4-6 ? ?
> 6 ? ?
(levels in mmol/L)
If it’s within the normal range then check regularly – ideally until your patient is well on its way to a full recovery.
Lactate levels may start increasing in response to hypoperfusion before the patient starts showing overt signs of deterioration – the fuel in the generator hasn’t run out yet – which makes it a very useful monitoring tool to detect problems early on.
We find a six to eight-hourly check in very sick patients will pick up deterioration fast enough to give you time to react, while a twice-a-day check in more stable animals will suffice.
Elevated
If it is increased at any point, you need to focus immediately on trying to reduce it.
This usually means starting with fluid boluses for shock. Recheck lactate one hour after initiating the appropriate therapy. Your goal is for the lactate value to be reduced by approximately 50% within one to three hours (ideally one hour) of initiating therapy. If it’s coming down nicely then keep checking every two to three hours.
You want it to be back to normal within 24 hours (48 hours max).
Nothing working?
If it has not decreased as expected – or, especially, if it increases despite treatment – it means things are going seriously wrong. At this stage you need to:
- Devote all your attention on trying to find and correct the underlying cause while you adjust your emergency therapy to address hypoperfusion.
- Speak to the owners.
- If you do not have the time, facilities or experience to deal with shock cases, you need to consider referring the patient to a specialist centre urgently for stabilisation, if this is an option available to you.
Remember…
Intense exercise, muscle tremors and seizures are associated with anaerobic muscle activity and can, therefore, cause significant increases in lactate levels (the cause of the “deep burn” when you’re dying in that CrossFit class). This can also occur when a patient resists when you are taking blood, or starts trembling in fear the moment it walks into the clinic, which can cause misleading lactate results.
Puppies can have a higher “normal” level of plasma lactate up to seven months of age.
Anaemia does not generally cause hyperlactataemia unless it is very severe, but by this stage you shouldn’t need lactate to tell you the patient is in serious trouble.
Having said that, normal lactate levels that suddenly start climbing in a hospitalised “stable” anaemic patient could be the push you need towards giving that blood transfusion.