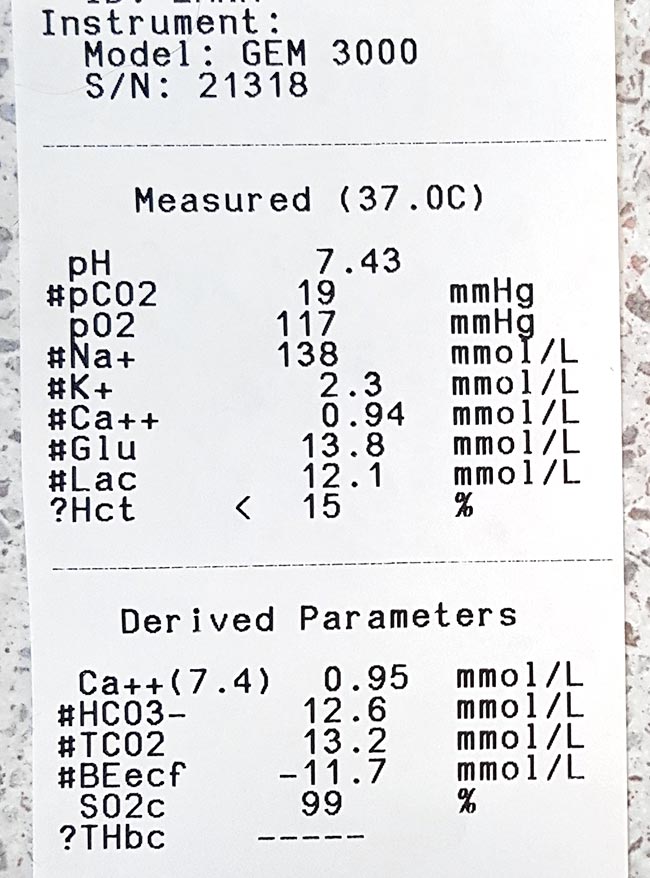
Ionised hypocalcaemia (iHCa) is a well-known electrolyte abnormality in critical human patients, which is also beginning to be recognised in our critical feline and canine patients.
The exact mechanism for the development of iHCa is still unknown – making prevention difficult, if at all possible. Controversy also exists as to whether treating iHCa is of any benefit, especially in non-clinical cases.
Despite these issues, serum concentration is proving to be an accurate prognostic indicator for the morbidity and mortality rates of some of the more critical patients.
Research
Over the past 30 years, significant resources have been put into trying to demystify the pathophysiological causes of iHCa in critically ill people; however, the exact mechanisms are still to be determined.
Some proposed mechanisms include:
- abnormal parathyroid hormone secretion or function
- abnormal vitamin D synthesis or function
- hypomagnesaemia
- calcium chelation
- alkalaemia
- calcium sequestration in tissue or cells
- an increase in calcitonin precursors (procalcitonin)
In a canine study where endotoxaemia was induced, it was found hypovitaminosis D was associated with iHCa (Holowaychuk et al, 2012).
Veterinary studies
The true incidence of iHCa in critically ill canine and feline patients is yet to come to a consensus, due to the limited veterinary studies.
In one retrospective study, 90% of 55 cats with septic peritonitis was reported to have iHCa (Kellett-Gregory et al, 2010), while only 24% of septic dogs (n=58) was reported to have iHCa (Luschini et al, 2010).
Regardless of the true incidence, the commonness of this change questions whether a need exists to treat iHCa, especially cases in the mild or non-clinical categories.
No consensus
At this stage, no consensus exists to either support or prohibit the treatment of hypocalcaemia in critically ill patients.
Well-designed prospective studies are scarce in human literature and non-existent in the veterinary field; no evidence-based guidelines are available for treatment.
Based on logic, arguments for the administration of calcium to critically ill patients include:
- iHCa during hospitalisation is a negative predictor for morbidity and mortality of patients.
- Hypocalcaemia can cause decreased myocardial contractility.
- In hypotensive patients dependent on vasopressors or inotropic agents, the supplementation of calcium may be beneficial.
Arguments against calcium supplementation include:
- Calcium accumulation within cells predisposes to hypoxia and ischaemia-reperfusion injury.
- Increased mortality in experimental models of sepsis when calcium is supplemented, on top of the lack of evidence to support this act.
Prognostic use
Serum calcium concentrations – or, rather, the trend of it in hospital – appears to be of valuable prognostic indicators.
Kellett-Gregory et al (2010) found although no direct associations existed between the presence or severity of iHCa at the time of patient admission, a positive correlation existed between the lowest iCa post-hospitalisation, and the length of hospitalisation and duration of intensive care stay.
Of the cats that had iHCa, those that failed to return to a normal ionised calcium (iCa) during hospitalisation had a significantly lower rate of survival to discharge. Interestingly, iHCa was not associated with the status of hypotension, coagulopathy or arrhythmias, so cannot be used to predict the occurrence of these.
These findings were echoed by Luschini et al (2010), where low mean ionised calcium and lowest documented ionised calcium concentrations were found to be associated with a poor outcome. The severity and duration of iHCa appears to be important in determining prognosis in these patients.
Conclusion
Controversy exists regarding whether treatment of mild iHCa in critically ill patients is recommended; however, one thing we now know is serum iCa concentration is a reliable predictor of mortality and morbidity in canine and feline patients.
References
- Kellett-Gregory LM, Mittleman Boller E, Brown DC and Silverstein DC (2010). Ionized calcium concentrations in cats with septic peritonitis: 55 cases (1990-2008), Journal of Veterinary Emergency and Critical Care 20(4): 398-405.
- Holowaychuk MK, Birkenheuer AJ, Li J, Marr H, Boll A and Nordone SK (2012). Hypocalcemia and hypovitaminosis D in dogs with induced endotoxemia, Journal of Veterinary Internal Medicine 26(2): 244-251.
- Luschini MA, Fletcher DJ and Schoeffler GL (2010). Incidence of ionized hypocalcemia in septic dogs and its association with morbidity and mortality: 58 cases (2006-2007), Journal of Veterinary Emergency and Critical Care 20(4): 406-412.