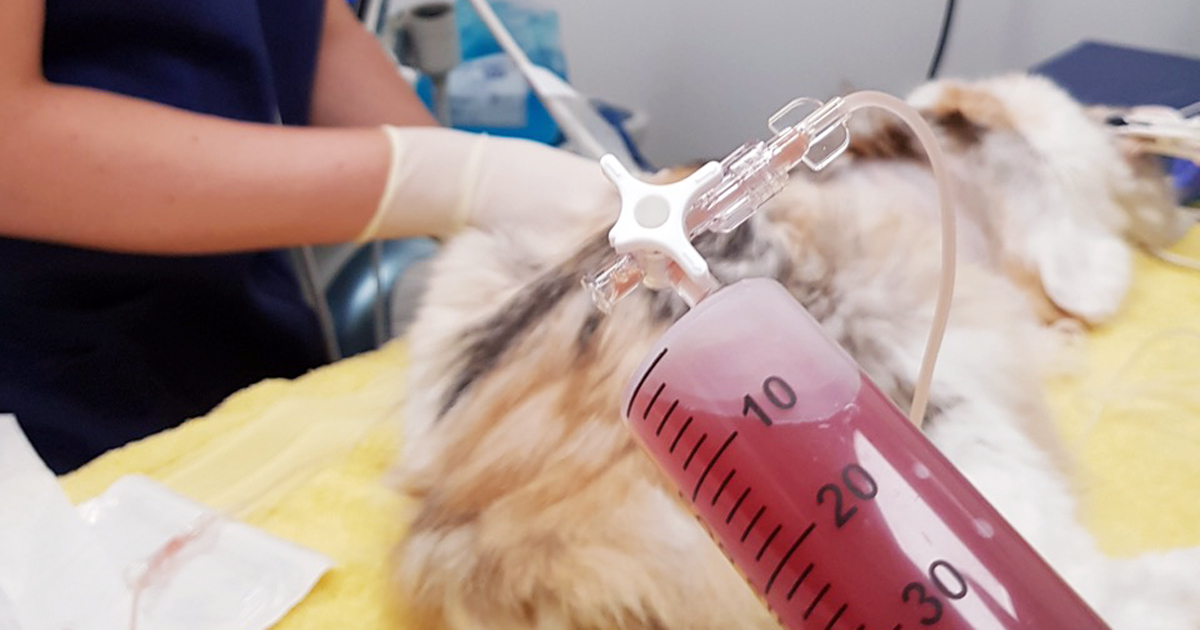
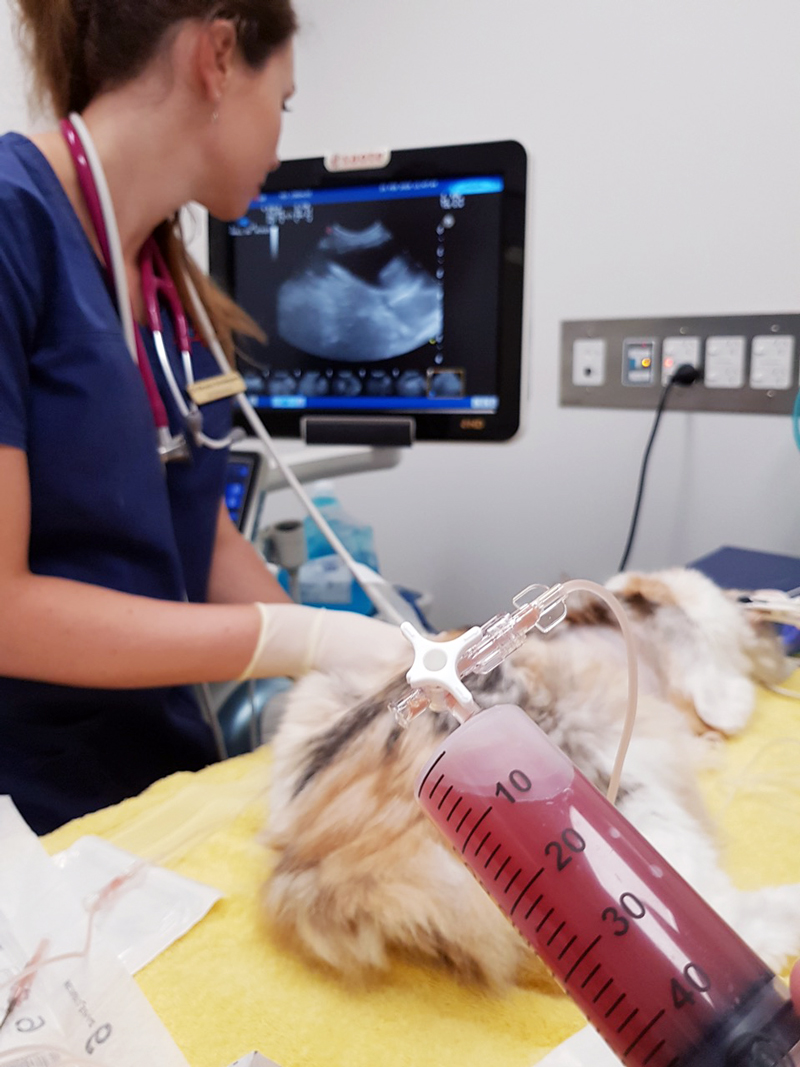
Last week we gave some hints and tips about how to perform a thoracocentesis. This week we look at what to do with the sample you collected and where to go to next.
Looking at the sample is not enough, there are several things you need to do to make sure you are getting the most information from the collected sample. This includes:
- Fluid cell counts
- Total protein assessment
- Packed cell volume
- Glucose
- Lactate (if it is an exudate)
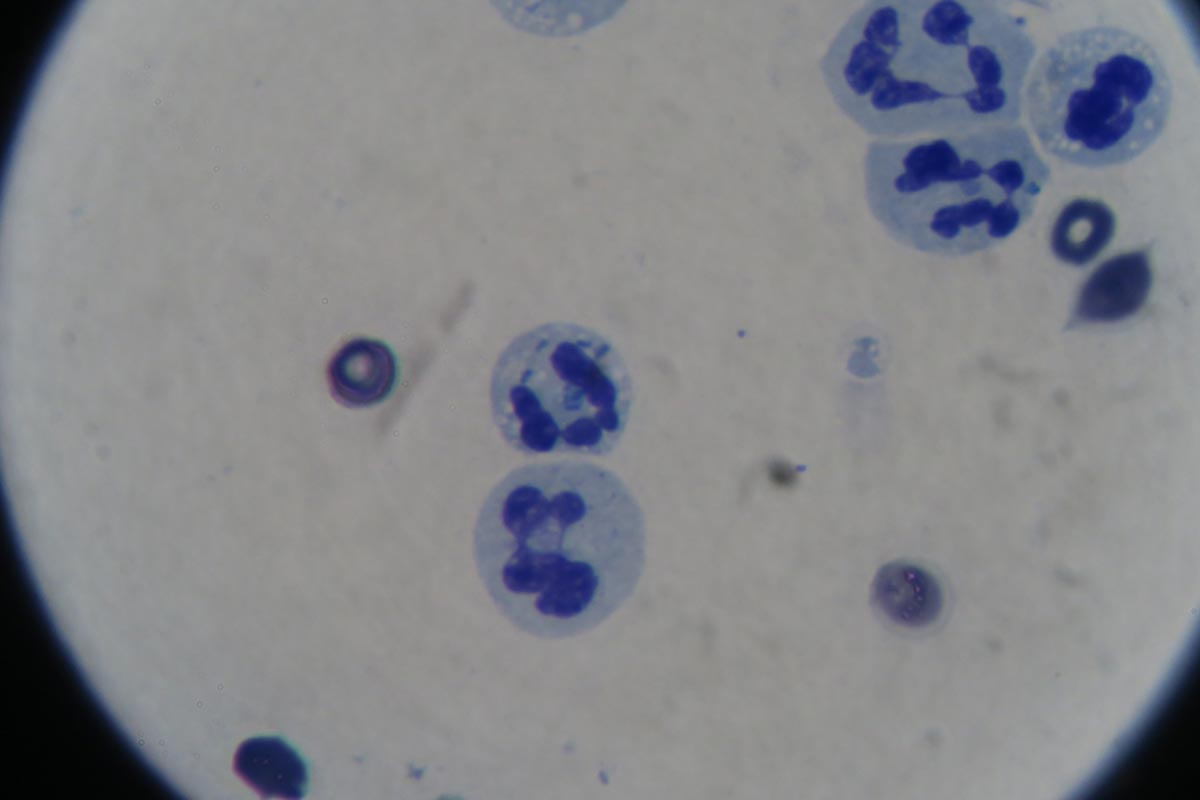
- In-house cytology
- Collect a sample for culture and sensitivity, and also external cytology assessment
With this information you can narrow down your list of differentials; often enough it can give you a diagnosis.
Here is the list I use. Note, it is not exhaustive and assumes you have taken three-view thoracic radiographs as part of the initial diagnosis.
Transudate
-
Haemorrhagic effusion. Clear appearance – characterised by low protein and low cellularity
- Transudates are caused by reduced oncotic pressure
- Total nucleated cell counts = <0.5x10e9/L
- Total protein = <25g/L
Differentials to consider
- Liver disease
- Protein-losing nephropathy
- Protein-losing enteropathy
Additional diagnostics
- Cytology and culture of fluid
- Haematology and biochemistry
- +/- dynamic liver testing
- Urinalysis, urine protein/creatinine ratio, culture and sensitivity
Modified transudate
- Yellow/serosanguinous/cloudy appearance
- Caused by increased hydrostatic pressure leading to passive leakage of proteins and fluid into the pleural space
- Total nucleated cell counts = 3.5-5x10e9/L
- Total protein = variable, ~25-50g/L
Differentials to consider
- Increased capillary hydrostatic pressure and pericardial disease
- Diaphragmatic hernia
- Neoplasia
- Lymphatic obstruction, such as neoplasia, diaphragmatic hernia and abscess
- Increased permeability of vessels (blood and lymphatics), such as FIP
Additional diagnostics
- Cytology and culture of fluid
- Haematology and biochemistry
- Cardiac auscultation and ultrasound
- +/- CT
Exudate
- Turbid appearance – Very proteinaceous liquid, froths when shaken
- Fluid is a mix of plasma and inflammatory mediators, and is caused by either septic or aseptic inflammation
- Total nucleated cell counts = >3.0x10e9/L
- Total protein = >30g/L
Aseptic exudate
- Non-degenerate neutrophils and activated mesothelial cells predominate
- Non-infectious cause
Differentials
- Inflammation: FIP (can have high globulins), liver disease, lung torsion and hernia
- Neoplasia
Additional diagnostics
- Haematology and biochemistry
- Cytology and culture of fluid
- +/- ultrasound/CT
- Further testing for FIP
Septic exudate
- Degenerate neutrophils predominate: nuclear swelling and pale staining
- Intracellular or extracelluar microorganisms
- Culture and sensitivity: aerobic and anaerobic
- Pleural fluid [glucose] < serum [glucose]
- Pleural fluid [lactate] > serum [lactate]
Differentials to consider
- Ruptured abscess
- Foreign body inhalation or penetrating injury
- Fungal infection
Additional diagnostics
- Haematology and biochemistry
- Cytology and culture of fluid
- +/- ultrasound/CT
Chyle
Opaque (milky) to pink.
Differentials to consider
- Rupture or obstruction of lymphatic flow
- Neoplasia, traumatic and idiopathic
- Secondary to heart failure (especially in cats)
- Pseudochyle (usually formed by lymphoma)
Additional diagnostics
- CBC and biochemistry
- Cytology and culture of fluid
- Fluid [TAG] > serum
- Large number of lymphocytes and other inflammatory cells
- +/- ultrasound/CT
Haemorrhage
- Red blood cells
- True haemorrhagic; for example, not iatrogenic: should not see platelets or erythophagocytosis on smears and sample should not clot
- Time frame
- Assess history
- Compare fluid PCV/total protein (TP) to peripheral PCV/TP:
- <1% – non-significant
- 1% to 20% – neoplasia, trauma, pneumonia
- >50% – haemothorax
- Other tips:
- If PCV/TP is similar = recent bleed, if PCV is low and TP normal = chronic
- If PCV is increasing or is higher than peripheral then active bleeding
- Presence of erythrophagocytosis = chronic
Differentials to Consider
- Trauma
- Neoplasia
- Coagulopathies
- Ruptured granuloma
Diagnostics
- Activated clotting time, activated partial thromboplastin time, prothrombin time, blood smear and other coagulation tests, see “coagulopathy”
- Blood smear
- CBC and biochemistry
- +/- ultrasound/CT
Good luck with your next thoracocentesis. I hope this information was useful.