The causes of hyponatraemia can be divided into three major categories, based on serum osmolality. This is further divided based on the patient’s volume status (Table 1).
Most patients we see in clinic fall into the hypovolaemic category, except patients with diabetes mellitus.
| Table 1. Causes of hyponatraemia based on osmolality and volume status (from Guillaumin and DiBartola, 2017). | ||||
|---|---|---|---|---|
| Hypo-osmolar | Hyperosmolar | Normo-osmolar | ||
| Hypovolaemic | Normovolaemic | Hypervolaemic | ||
|
Gastrointestinal fluid loss
Third-space fluid losses
Shock
Hypoadrenocorticism (Addison’s disease)
Renal insufficiency
Excessive diuretic administration
Salt-losing nephropathy
Cerebral salt wasting syndrome
|
Syndrome of inappropriate antidiuretic hormone secretion (SIADH)
Hypotonic fluid administration
Hypothyroidism
Glucocorticoid insufficiency
Psychogenic polydipsia
Reset osmostat (SIADH type B)
|
Congestive heart failure
Acute or chronic renal failure
Nephrotic syndrome
Hepatic cirrhosis
Accidental ingestion or injection of water (water intoxication)
|
Hyperglycaemia
Mannitol
Severe azotaemia
|
Hyperlipidaemia
Hyperproteinaemia
|
Common causes
In dogs, the three most common causes of hyponatraemia are:
- gastrointestinal (GI) fluid loss
- third-space fluid loss
- fluid shift from intracellular fluid to extracellular fluid (ECF) as a result of hyperglycaemia
In cats, the three most common causes of hyponatraemia are:
- urologic diseases
- GI fluid loss
- third-space fluid losses
In most patients, more than one pathophysiologic factor is likely to be contributing to the hyponatraemia.
Circulating volume
Hypovolaemic patients – those with, for example, GI losses, hypoadrenocorticism, renal losses and haemorrhagic shock – have a reduced effective circulating volume. ECF contraction triggers antidiuretic hormone (ADH) secretion, which leads to increases in free water absorption and thirst, and results in dilution of the serum sodium concentration. Aldosterone secretion is reduced in hypoadrenocorticism, so an overall reduction in sodium reabsorption compounds the problem.
Hypervolaemic patients are those with an increased fluid retention state, such as:
- congestive heart failure (pulmonary oedema)
- advanced hepatic failure (ascites, third-space fluid)
- renal failure
- free water ingestion
Congestive heart failure patients have a reduced cardiac output and, therefore, a decreased effective circulating volume, despite the presence of the extra fluid status. Renin-angiotensin activation leads to release of ADH and aldosterone, resulting in sodium and free water reabsorption, and increased thirst. Both lead to an excess of free water retention.
Advanced hepatic (cirrhosis) or renal failure (nephrotic syndrome) both result in hypoalbuminaemia, leading to fluid shifting into the interstitial space and third space, reducing effective circulating volumes. This leads to activation of ADH to increase free water reabsorption, to restore the circulating volume in the face of existing hypervolaemia and hyponatraemia.
Diabetic patients
Moderate to severe hyperglycaemic diabetic patients can be either hyperosmolar or normo-osmolar, depending on the serum blood glucose concentration. Hyponatraemia occurs when water shifts from the intracellular fluid to the ECF down the osmotic gradient, diluting the serum sodium content.
Despite this osmotic shift, not all diabetic patients develop hyponatraemia. Glucosuria also causes also causes a renal osmotic shift, sometimes resulting in urine water loss in excess to sodium. This offsets the hyponatraemia – in some cases, hypernatraemia results.
Treatment
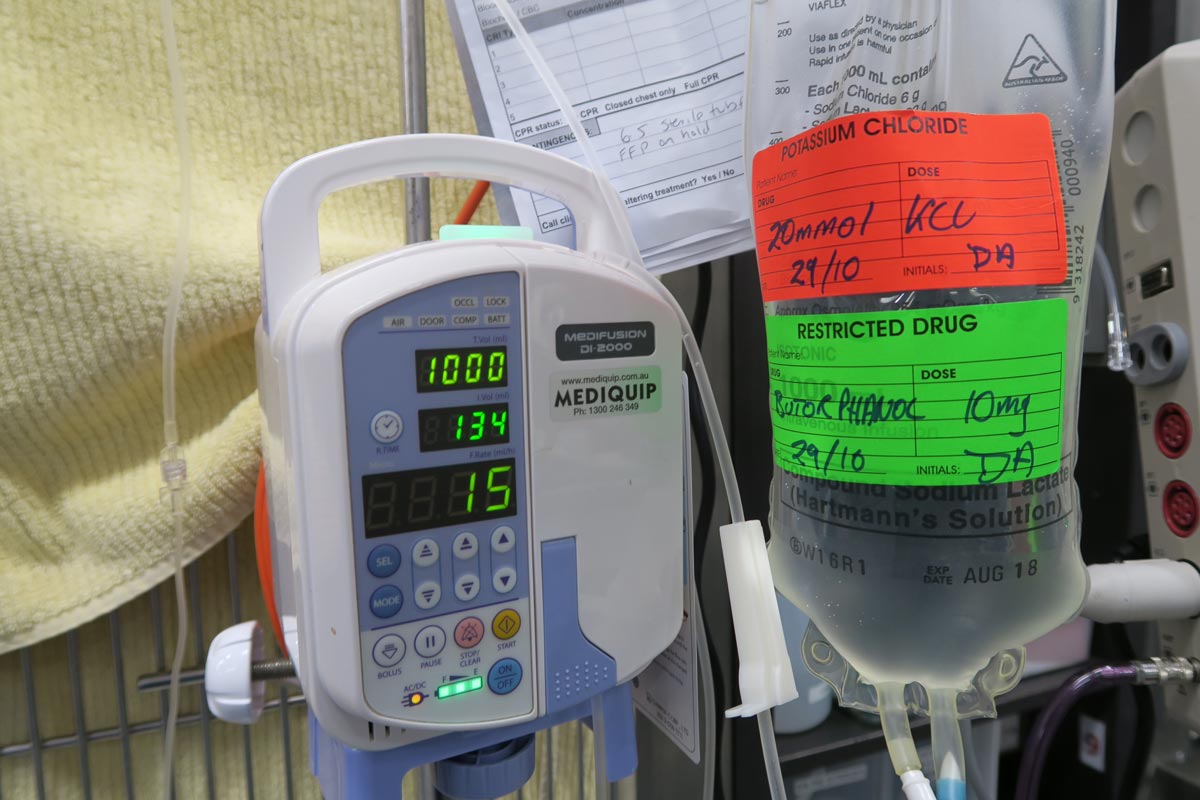
Treatment of hyponatraemia hinges on how quickly it developed and the volume status of the patient. The rule of thumb is to correct hyponatraemia slowly – not exceeding 0.5meq/L/hr – especially in chronic cases, or cases where the duration of hyponatraemia is unknown. Keeping to this rate is paramount until serum sodium concentration reaches 130meq/L.
In acute patients with severe clinical signs, such as seizures, some clinicians may choose to use a higher rate of 1meq/L/hr to 2meq/L/hr until clinical signs resolved.
It should be emphasised, once again, this rate should never be used in chronic patients, patients with an unknown duration of hyponatraemia, or where frequent serum sodium concentration cannot be monitored. The rapid correction of hyponatraemia can lead to osmotic demyelination syndrome (myelinolysis).
Its effect will not be apparent until three or four days after therapy, and can result in neurological abnormalities such as:
- weakness
- ataxia
- dysphagia
- paresis
- coma
For that reason, frequent electrolyte measurements are required, starting hourly then once a suitable rate of increase has been established and less frequently thereafter.
- Part 3 will look at how to correct patients with hyponatraemia.
Reference
Guillaumin J and DiBartola SP (2017). A quick reference on hyponatremia, Veterinary Clinics of North America: Small Animal Practice 47(2): 213-217.

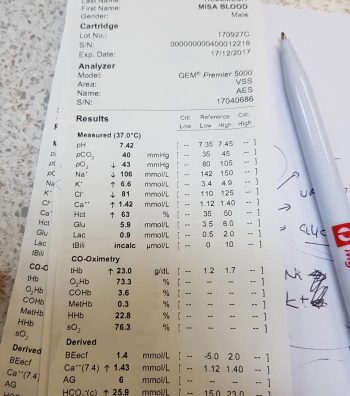








 For cases with a normal pH, we need to determine which category it falls into:
For cases with a normal pH, we need to determine which category it falls into: Once the primary disorder has been identified, we need to look at whether a secondary disorder is also present and, if so, whether this is the result of compensation or a true mixed process.
Once the primary disorder has been identified, we need to look at whether a secondary disorder is also present and, if so, whether this is the result of compensation or a true mixed process.