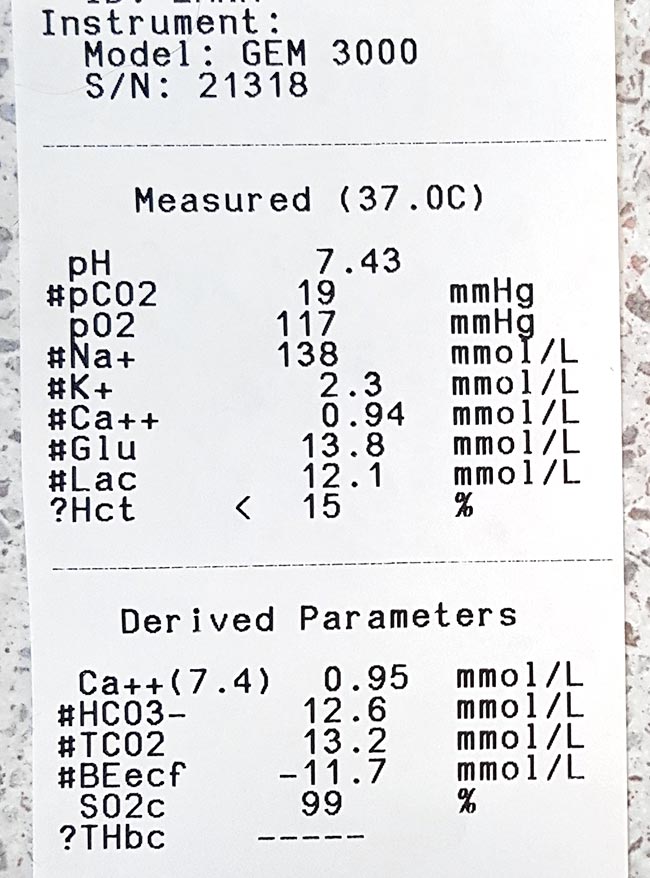
They’re not eating. They’re not drinking. And they’re turning their nose up at everything you offer. Add post-operative food aversions to a need to initiate voluntary fluid and food intake, and it can feel like an uphill battle of wills.

What have you got that supports hydration, is easily digestible and is palatable enough to encourage eating and drinking in your hospitalised and at-home patients?
Furr Boost has the answer…
It started with the bladder
Encouraging her poorly beagle Phoebe to drink is a battle Louise Toal faced after her vet diagnosed urinary issues. She just wasn’t interested in drinking. Luckily for Phoebe, Louise is a Food Technologist and began experimenting with protein shakes for dogs, to bolster hydration and combat inappetence. This is when Furr Boost was born.
It’s more than a hydration drink; it supports healthy digestion, immunity, metabolism and anxiety… plus, it’s delicious.
Furr Boost has taken the pet market by storm and even braved the Dragon’s Den dragons. Now, this highly palatable, nutritious hydration solution has hit your wholesalers for use in clinic!
What is Furr Boost?
In short, it’s a nutritional superfood smoothie for your canine and feline patients. Packed with 100% natural, functional ingredients and no fillers. It’s a low fat, but irresistible blend of meat, fruit and vegetables to use as a drink, food topper or boredom breaker.
Low in purine, protein, sodium and phosphorous, it’s ideal for encouraging inappetent patients to eat and hydrate post-operatively, during hospitalisation, and at home.

Hydration
Water… but tasty! More than 75% moisture content to replenish lost fluid and nutrients.

Healthy metabolism
Contains low-fat protein sources plus a blend of oils to provide energy for metabolism and to support a normal recovery.

Immunity
Packed with ingredients to support a robust immune system.

Digestion
Pre-biotics and dietary soluble fibre aid normal digestion and maintain healthy gut motility.

Anxiety
B vitamins and water combat anxiety. Use frozen or on lick mats to distract anxious patients.

Skin and coat
Full of naturally occurring fatty acids and omega 3 and 6 for hydrated, healthy skin and a glossy coat.
Furr Boost case study – meet Ozzy
Meet Ozzy the Saluki who was diagnosed with canine meningitis on 24 June, weighing in at 16Kg and was stabilised by the wonderful medical team who treated him.

Owner Jayne was very worried for her beloved pet and could see the visible signs of not only his weight loss after his ordeal, but also his mood.
Jayne said: “When he came home he was so skinny, dehydrated and reluctant to engage with food and drink despite what I tried. Then I discovered Furr Boost and imagine my delight when he absolutely lapped it up with real enthusiasm. He absolutely loves it!
“I was struggling to get him interested in eating anything to try and build him up while he was taking seven steroids a day. A Furr Boost a day has aided his progress I am sure. He is still on four steroids a day but his weight is now a healthier 22kg. Hoping for dose to reduce to two per day next week.
“It’s been a long job and he is very flat with the medication, which hopefully will start to improve. I was just so grateful to find your drink when things were looking very grim for him.”
While Furr Boost is mainly for hydration, the all-natural and human-grade ingredients are used to entice the dog to drink or eat if used as a topper. Our highly palatable formula is irresistible to even the fussiest of dogs and is packed with oils and nutraceuticals to get dogs back on their feet. Our drinks can be used in their pourable form or, for dogs who are not drinking, watered down by up to 50% to push fluids*.
* We recommend dogs drink 50ml of fluid per body weight and in extreme cases fluid intake should always be monitored.
Where does Furr Boost fit into your practice?
Whether it’s in the kennels, in recovery, or to recommend to your clients, Furr Boost is a highly palatable, convenient source of hydration and nutrition. Give as a drink to replenish lost fluids and nutrients, or feed it to encourage voluntary enteral eating and drinking, and to support normal gut motility.
If patients are food-averse, or the palatability of recommended diets is causing food refusal, add Furr Boost as a topper to soften kibble and entice their tongues back into action, with a range of lip-smackingly delicious flavours.




Furr Boost is suitable for:
- all dogs and cats over the age of eight weeks
- pregnant and whelping bitches and queens
- recovery
- dogs requiring a low sodium, protein, phosphorous or purine diet
- maintaining hydration
- supporting weight management or low-fat diets
- encouraging inappetent dogs
- end-of-life support
- distraction and enrichment, particularly for anxious or immobile patients
Get your free sample at The London Vet Show
Have you got a patient in mind that Furr Boost could support? Would you like to test it in your clinic?
Come along to stand F66 at the London Vet Show on 14-15 November 2024 to grab your free carton!



Not going to be at the show?
No worries, Furr Boost is now available via MWI Animal Health, Covetrus, and IVC Evidensia, or you can order directly at www.furrboost.com/veterinary where our team is also available to answer any questions.