Vets have to handle a variety of difficult situations, from delivering bad news to addressing financial difficulties. But when it comes to tackling client grief, awkwardness or anger, it’s a bit like that old children’s book, We’re Going on a Bear Hunt – we can’t go over, under or around it – we’ve got to go through it!
I recently had a three-hour communications practical, during which my fellow students and I were tossed into a randomly selected clinical scenario designed to help us deal with uncomfortable areas of future practice. These included such topics as:
- discussing the needs for euthanasia
- apologising for clinical mistakes
- reasoning with angry farmers when their herds came back TB-positive
Role play

All scenarios were conducted with real actors portraying the clients – and although we’d been given a list of topics to revise if we wanted – any revision turned out to be next to useless as the exercise was less about what you knew, and more about how you dealt with people.
Personally, I find this type of practical as rewarding – if not more so – than our clinical skills work. We have countless opportunities to practise suturing, spaying and catheterising in the labs at school, and in final-year rotations, but dealing with the raw side of client communications is the one thing we never actually get to experience until we’re suddenly in the driver’s seat.
It’s completely understandable – nobody wants a student present at their most vulnerable moments. If my own pet was being put down, I’d want the comfort of an experienced vet doing the job and walking me through it from start to finish.
Learning experiences
That’s why I think communication practicals are so incredibly useful, and it’s a real shame that I’ve only had three in my course so far. It can be difficult to learn from and build upon experiences spaced years apart from one another, and I feel incredibly sorry for the year before me (while I was intercalating) who had to do their scenarios over Zoom.
One of the most valuable lessons that these practicals have taught me, is not to be afraid of emotion.
It can be difficult – especially when a very valid defence mechanism for many medical professionals is to distance yourself from it at all costs – but there are moments when all is required of you is simply to be there, to listen and understand.
Just be there
There’s no textbook in the world that can teach you that. When a client (or an actor pretending to be a client) is crying in front of you, you want to fix their grief because fixing things is, quite literally, your profession.
Your instinct is to talk and fill the silence, but instead, you need to wait for them to process the moment and then be there to answer their questions.
Some things you can’t fix and you can’t work around – you just have to go through them.


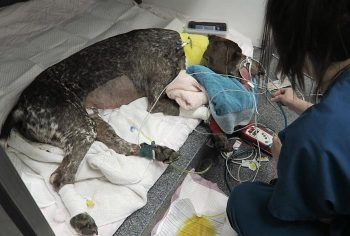
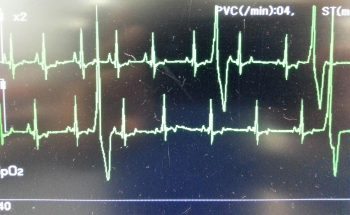
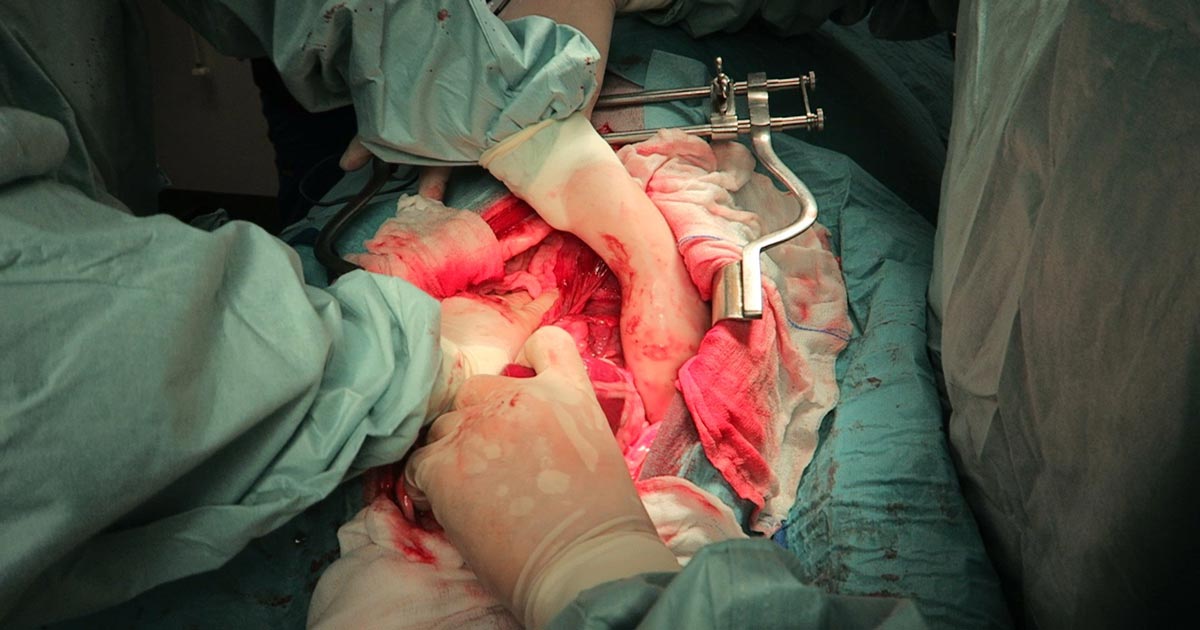

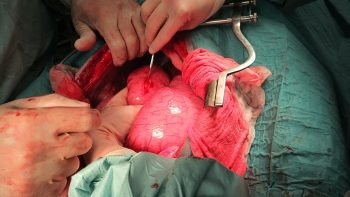

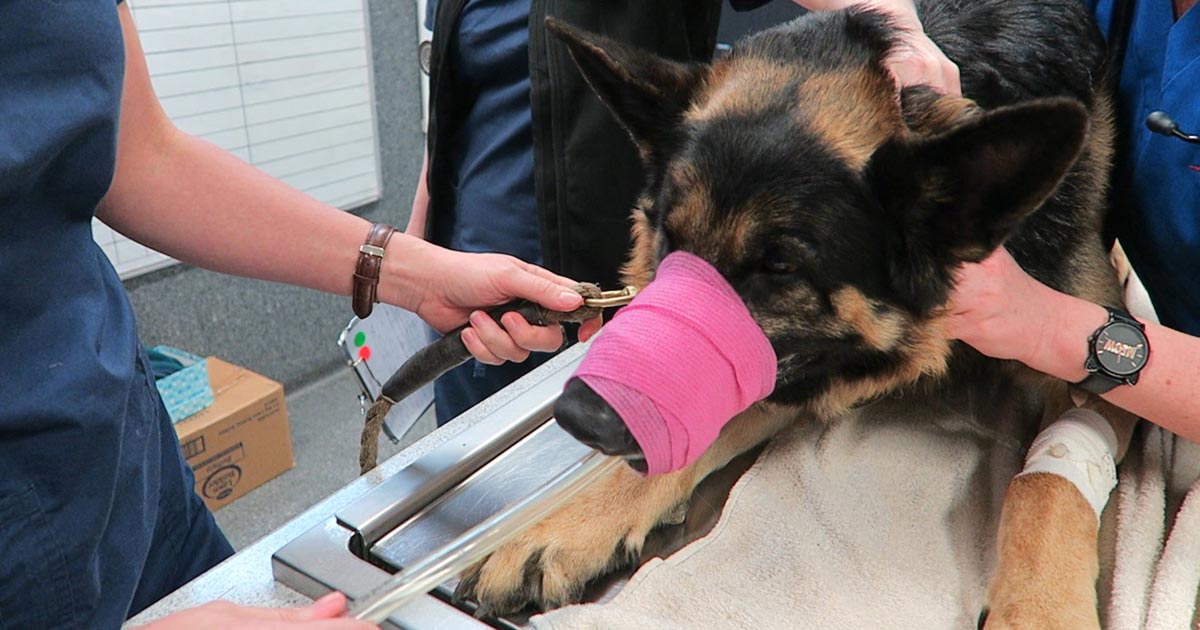

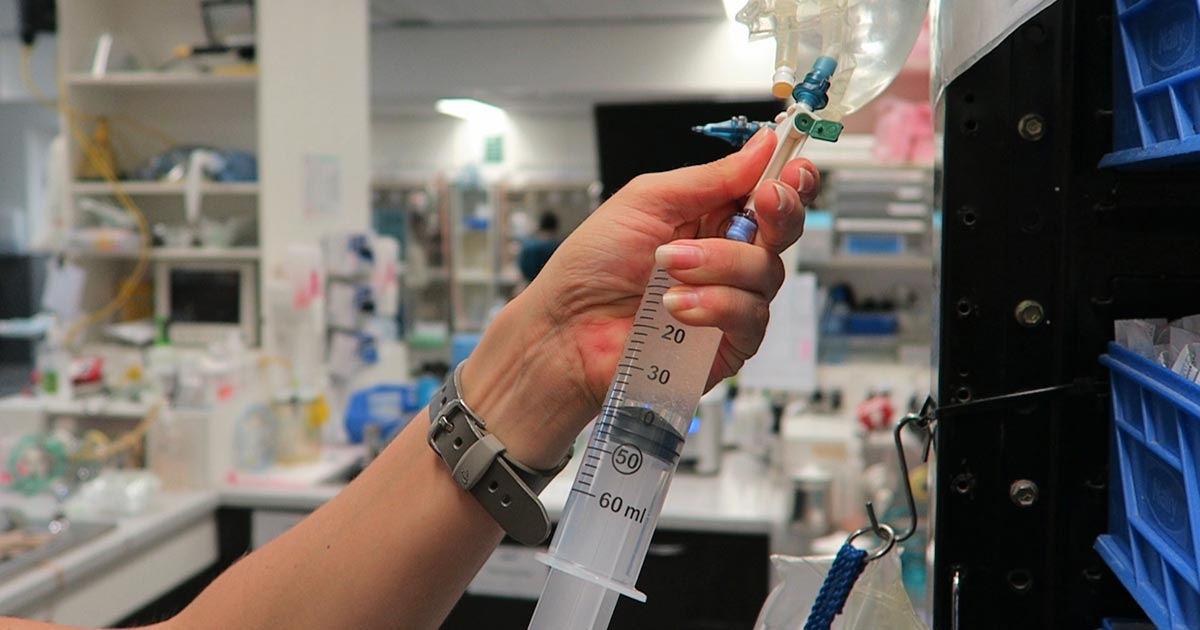

 Numerous online calculators can determine whether a toxic dose has been consumed and they are a great place to start.
Numerous online calculators can determine whether a toxic dose has been consumed and they are a great place to start.