As discussed in part one of this article (VT48.05), cutaneous and renal glomerular vasculopathy (CRGV) is variably associated with development of acute kidney injury (AKI).
AKI is defined as a rapid reduction in renal function, leading to an inability to regulate fluid balance, electrolyte concentrations and acid-base status (Schrier et al, 2004). Glomerular filtration rate (GFR) decreases, eventually leading to increased creatinine concentration and, possibly, reduced urine production (Bellomo et al, 2012).
It is important to recognise a dog developing AKI will not be azotaemic initially. An increase in serum creatinine concentration of 26.4µmol/L above baseline within a 48-hour period, and/or urine production of lower than 1ml/kg/hr or anuria over a 6-hour period, should be recognised as indicating early AKI (International Renal Interest Society [IRIS] AKI grade one; Brown, 2016). Care should be taken to avoid misinterpretation of results if different laboratories/biochemistry analysers are used.
If a suspected CRGV dog is not azotaemic and not thought to have AKI, monitoring for the potential development of AKI should be continued. The timing of the development of AKI in CRGV patients is variable, but, when it develops, it is most commonly identified 3 to 4 days after the skin lesions appear (range 0 to 10 days; Holm et al, 2015).
The onset of AKI in CRGV patients is sometimes preceded by the development of mild to moderate thrombocytopenia (Anderson Moores Veterinary Specialists, unpublished data). This is considered to be the result of the underlying thrombotic microangiopathy (TMA), as the formation of thrombi and reduction of renal glomerular blood flow triggers the initiation and extension phases of AKI.
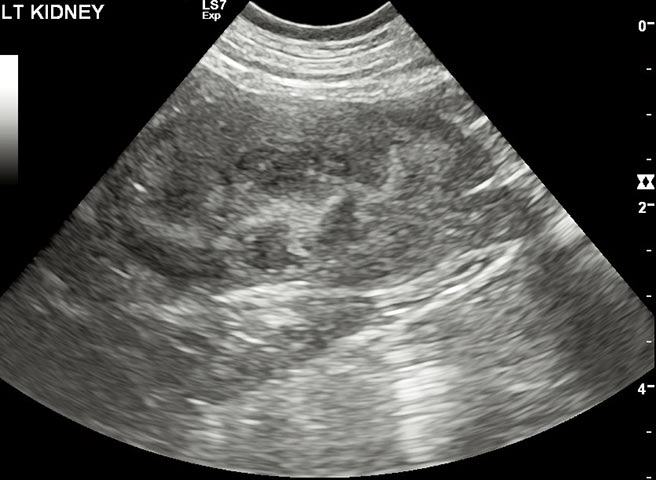
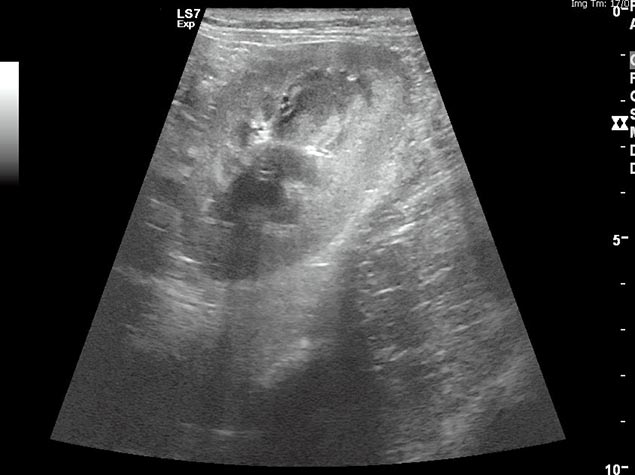
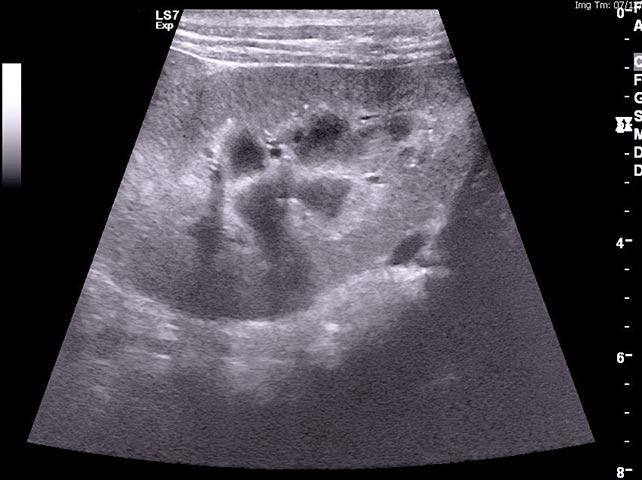
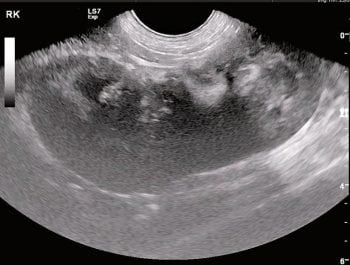
It is important to remember azotaemia (serum creatinine concentration above the top of the reference range) is generally not identified until 75% of renal function has been lost (Braun et al, 2003). If AKI develops, efforts should be made to identify the underlying cause. Abdominal imaging can be useful to exclude renal neoplasia – CKD and pyelonephritis, for example (Figure 1) – and testing for hypoadrenocorticism and leptospirosis may also be useful to exclude these as possible causes of azotaemia.
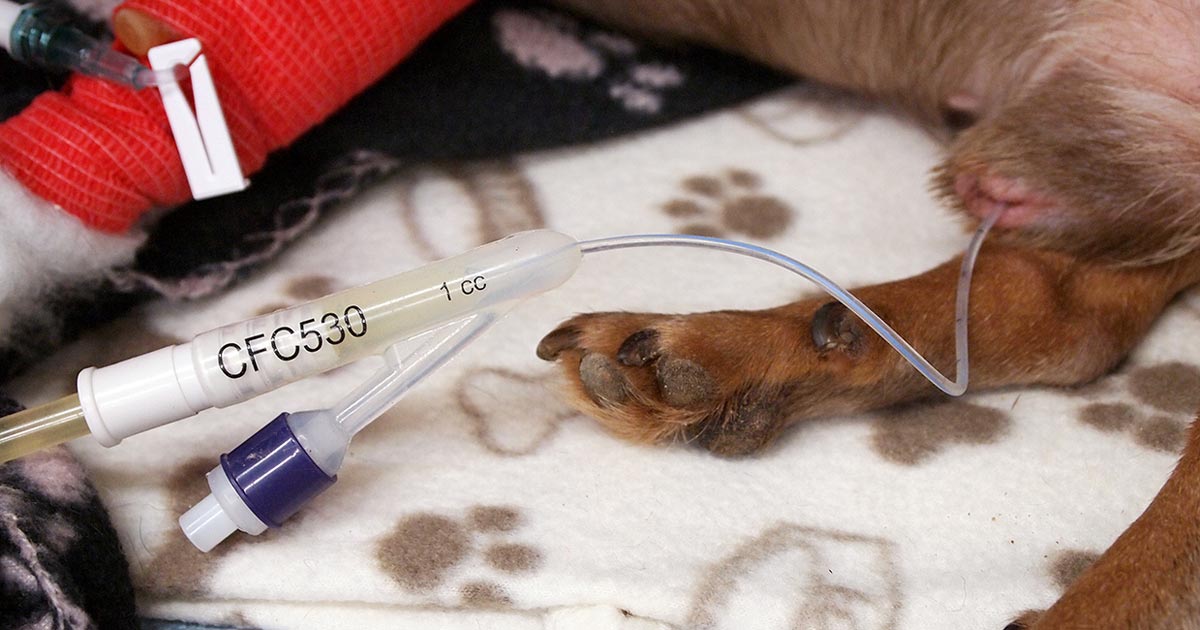
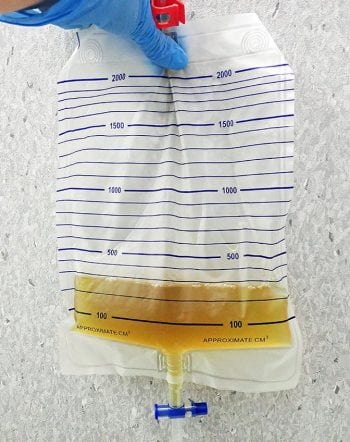
Management of AKI should be aimed at maintaining optimal fluid, acid-base and electrolyte status. An indwelling urinary catheter (Figure 2) enables accurate measurement of urine output, which helps the clinician to determine IV fluid therapy (IVFT) requirements. This facilitates ensuring the patient does not become either volume depleted if polyuria develops (which could cause further renal damage) or volume overloaded if oligoanuria develops. Oliguria is defined as urine production of lower than 1ml/kg/hr and anuria as no urine production over a six-hour time period (IRIS, 2016).
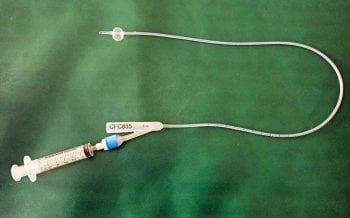
Placement of a Foley urinary catheter (Figure 3) can be achieved in most male dogs without the need for sedation; however, females can be more challenging and light sedation may be required (taking care with systolic blood pressure, to avoid adversely affecting GFR). Aseptic technique should be used (Smarick et al, 2004) and a urine collection bag should be attached to achieve a closed system (Figure 4).
Management
Fluid therapy
The deficit is (7/100)×20 = 1.4L or 1,400ml.
To correct this over 6 hours: 1,400/6 = 233ml/hr.
Maintenance requirement is usually estimated as 50ml/kg/d: (50×20)/24 = 41.7ml/hr.
If the dog vomits and this is estimated to be 40ml, but no diarrhoea occurs, then the loss is 40ml. This would give a total fluid rate for the first hour as 233 + 41.7 + 40 = 314.7ml/hr (15.7ml/kg/hr).
If the dog has no further vomiting or diarrhoea, the rate for the next 5 hours is 274.7ml/hr (13.7ml/kg/hr).
Thereafter, the rate would be calculated via the ”ins and outs” chart (Table 2) using urinary, insensible and other losses (such as vomiting and diarrhoea) to calculate the required rate.
Fluid therapy is the mainstay of management of patients with AKI. The initial rate of IVFT should be calculated based on the patient’s bodyweight and hydration status. The dehydration deficit should be corrected over four to six hours, and this deficit should be given in addition to maintenance requirements (50ml/kg/day) and any ongoing losses associated with vomiting and diarrhoea (Panel 1; Ross, 2011).
Generally, clinical signs of dehydration do not occur until approximately 5% body water has been lost. In the absence of clinical signs, a 5% deficit would be assumed. If clinical signs are present, dehydration should be assumed to be in the range of 7% to 12%, based on the severity of the clinical signs (Table 1; Tello and Perez-Freytes, 2017).
Rarely, the patient will be hypovolaemic and this should be corrected rapidly, via administration of 10ml/kg to 20ml/kg crystalloid fluid boluses, each given over 10 to 20 minutes and repeated as required based on heart rate, mucous membrane colour, pulse quality and (if possible) systolic blood pressure measurement. If concurrent, significant cardiac disease is present, a more cautious approach will need to be adopted (Mathews, 2006). Once the patient is adequately hydrated, if it is not oligoanuric, urine production should increase to between 2ml/kg/hr and 5ml/kg/hr.
If urine output is adequate, IVFT should be continued, calculating the rate to match ”ins and outs” (Table 2).
The total volume ”in” is calculated as the volume of fluids infused over the preceding hour, plus any fluids drunk or fed. The total volume out is calculated as urine produced over the preceding hour, plus insensible loss and any vomit/diarrhoea. Insensible losses are calculated as 0.5ml/kg/hr to 1ml/kg/hr and generally arise from the respiratory tract; therefore, patients that are panting will have a higher rate of insensible loss than those with a normal respiratory rate and effort. The total volume ”out” is subtracted from the total volume in to ascertain the fluid balance for that hour. The total volume out over the hour dictates the volume of fluids to be infused over the next hour.
Physical examination and close monitoring of bodyweight, vital parameters and systolic blood pressure should help with assessment of patient volume status. The following signs may suggest volume overload:
- Increased respiratory rate and effort.
- Presence of moist nasal discharge.
- Increasing bodyweight in excess of 5% to 10% above baseline (this should be monitored every six hours).
- Increasing systolic blood pressure (again this should be monitored at least every six hours).
- Presence of peripheral oedema.
Serum electrolyte concentrations should be closely monitored. Serum potassium concentration should be monitored and IVFT composition adjusted to avoid development of hypokalaemia or hyperkalaemia. Rapid changes of serum sodium concentration should also be avoided due to the potential for development of adverse neurological sequelae.
Patients with indwelling urinary catheters are at risk for ascending urinary tract infection. Catheters should be kept clean and routine catheter care should be employed at least every eight hours (Smarick et al, 2004). Daily urine sediment examinations should also be performed to assess for evidence of urinary tract infection, allowing early intervention if detected. Urinary catheters should ideally be replaced every three days to minimise the risk of ascending infection (Ross, 2011).
Antiemetics
Nausea is common due to a combination of the central effects of uraemic toxins on the chemoreceptor trigger zone and possibly local uraemic gastritis.
Centrally acting antiemetics, such as maropitant, metoclopramide and dolasetron or ondansetron, are, therefore, often beneficial. Metoclopramide is generally most efficacious when used as a continuous rate infusion (CRI). Ondansetron and dolasetron can be used in patients whose signs are not controlled with maropitant and metoclopramide.
Analgesia
The skin lesions seen in CRGV patients are typically very painful. Abdominal discomfort may also be present, associated with AKI. Analgesia is, therefore, considered to be indicated, but careful attention should be given to avoid potentially nephrotoxic drugs or those that could interfere with GFR.

Opioids are generally considered appropriate and the drug choice and dose administered should be based on the patients’ clinical need.
Antacids
In humans, decreased renal function can lead to hypergastrinaemia (Dacha et al, 2015). Previously, it was thought this resulted in increased gastric acid secretion, but it is known these patients commonly have normal or low gastric acid secretion (Dacha et al, 2015). Hypergastrinaemia leading to increased gastric acid production and ulceration has also not been documented in dogs with azotaemia (Peters et al, 2005).
Nonetheless, vomiting is one of the most common signs of azotaemia in dogs with AKI (Ross, 2011). Antacid medication, such as famotidine (H2-receptor antagonist) or omeprazole (proton pump inhibitor), may, therefore, be beneficial and reduce the risk of oesophagitis developing in patients with severe vomiting and/or regurgitation.
Anti-hypertensives
Arterial hypertension commonly develops in patients with AKI, and can be both severe and relatively refractory to management in CRGV cases. If hypertension is documented, the patient’s volume status should be carefully assessed to ensure they are not overhydrated, as this could be contributing to hypertension.
Management is generally considered to be indicated if systolic blood pressure persistently exceeds 160mmHg to 170mmHg, due to the risk of damage to the retina, heart and CNS (IRIS, 2016).
Management may, however, be challenging. If oral medication can be used, amlodipine should be started. It is generally best to start at the low end of the dose range and titrate upwards as required to try to achieve a systolic blood pressure of 120mmHg to 160mmHg.
As angiotensin-converting enzyme inhibitors have been associated with worsening renal function in humans with CKD, hypertension and congestive heart failure (Chrysant et al, 1983; Weinberg, 1993), as well as being a risk factor for development of AKI in hospitalised patients (Hodgson et al, 2017), their use is generally not recommended for dogs with AKI.
Blood products
CRGV patients have at least two mechanisms by which significant anaemia may develop: thrombocytopenia, if severe, can lead to spontaneous bleeding and secondary blood loss anaemia, and microangiopathic haemolysis.
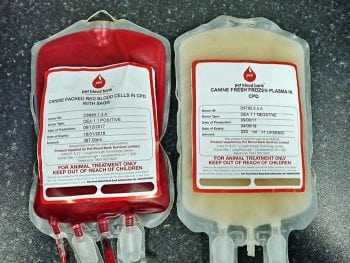
Blood transfusions (whole blood or packed red cells; Figure 5) can be considered for patients with clinical signs of anaemia attributable to either low PCV (generally lower than 20%) or rapidly falling PCV. Great care must be taken with volume status in oliguric patients, and any blood products must be factored into fluid balance calculations.
The role of plasma transfusion, or plasma exchange therapy (used in the management of some human TMA illnesses; Thejeel et al, 2016) remains unknown in dogs with CRGV.
Nutritional support
Patients that appear to be responding favourably to medical management for AKI may require nutritional support if calorie requirements are not being met over a period of several days; however, enteral nutrition will only be possible if the patient is either not vomiting, or if vomiting can be controlled via medical management.
As long as the patient does not have severe thrombocytopenia, a naso-oesophageal or oesophageal feeding tube can be considered. A readily digestible diet, which is not high in fat, should be chosen. No urgent requirement exists for the immediate initiation of a renal support diet, although this may become necessary if the patient makes a partial recovery and is left with residual CKD. Nutritional support will help to prevent the adverse effects associated with malnutrition, such as impaired immunity, cachexia, reduced cellular repair and impaired drug metabolism.
This intensive management should be continued, checking serum biochemistry daily, until the serum creatinine concentration has improved to a plateau. The creatinine may plateau within the reference range, but some patients remain azotaemic. Once the plateau has been reached, assuming the patient is doing well clinically, IVFT should be gradually weaned, usually by approximately 25% of the original rate over each 24-hour period.
Bodyweight and serum creatinine concentration should continue to be monitored during this process. It is not uncommon for patients to become more azotaemic again as the IVFT is weaned and, if required (based on clinical status), the IVFT rate can always be increased again. The weaning process can be repeated once clinical status improves.
If renal function fails to improve to a point where the animal could be medically managed at home (over days to weeks), or if it worsens, unfortunately, the patient would either require dialysis, or euthanasia may have to be considered.
Oligoanuria
If oliguria is identified in a well-hydrated patient (based on physical examination and systolic blood pressure measurement), the general recommendation is to initiate medical management to promote diuresis, although no controlled studies (in humans or animals) exist documenting an improved outcome associated with the use of diuretics (Nielson et al, 2015; Shah et al, 2016).
Furosemide is generally the first line treatment for management of oligoanuria associated with AKI. This can either initially be administered as a bolus of 2mg/kg; or a bolus of 0.66mg/kg, followed immediately by a CRI at 0.66mg/kg/hr (Adin et al, 2003). If a 2mg/kg bolus is not effective at producing diuresis within an hour, single incremental boluses (4mg/kg then 6mg/kg) should be administered.
If a bolus is effective, treatment can be continued with a CRI (0.3mg/kg/hr to 1mg/kg/hr) for 24 hours to 48 hours. If furosemide is ineffective, mannitol can be used, and is initially administered as a 0.25g/kg to 0.5g/kg bolus of 10% or 20% solution. If this is effective, a CRI (1mg/kg/min to 2mg/kg/min) can be continued for 12 hours to 36 hours. Mannitol should be used with caution in oligoanuric patients as it can worsen circulatory overload. If mannitol is ineffective, dialysis should be considered.
Options for dialysis
Peritoneal dialysis
Dialysis catheters have to be placed surgically, which may be problematic if patients are thrombocytopenic. Sterile handling of the catheter sites and wound dressings is required, and dialysis should be performed every one to two hours initially.
Commercial dialysate is available; however, in an emergency situation, a home-made solution can be used. If the patient improves, the interval can be gradually extended to every three to six hours. Peritoneal dialysis is labour-intensive and expensive, but nonetheless, likely to be cheaper than continuous renal replacement therapy (CRRT). Further detail is beyond the scope of this article, but a number of review articles are available (Bersenas, 2011; Ross and Labato, 2013).
CRRT
CRRT can be considered for oligoanuric cases not responding to medical management. This therapy is only available in the UK at the RVC. Not all cases would be deemed suitable candidates; therefore, each should be individually discussed regarding suitability, prior to onward referral.
Prognosis
Sadly, for dogs developing significant AKI, the prognosis appears to be extremely guarded. Many cases have been euthanised due to refractory oligoanuria. Some cases have developed seizure activity terminally, while, for others, owner concerns regarding financial constraints, likely poor prognosis, intercurrent disease, or behavioural issues have led to the decision to euthanise (Holm et al, 2015).
It, therefore, remains unknown whether with prolonged supportive care, including CRRT, any of these cases could eventually have recovered.
As mentioned in part one, cases remaining non-azotaemic, or developing mild AKI (IRIS grade one to two), have a much more favourable outlook and full recovery may be expected with appropriate management.
Renal histopathology
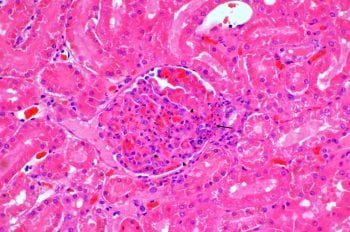
Confirmation of the diagnosis of CRGV relies on compatible renal, and occasionally dermal, histopathology. Given the relative contraindication for renal biopsy in an AKI patient (which would also frequently be thrombocytopenic), the definitive diagnosis is, therefore, generally only achieved postmortem.
Frequently identified abnormalities include fibrinoid necrosis of the glomerular arterioles with fibrin thrombi inside the glomerular tufts and capillaries, and tubular necrosis (Figure 6).
Disease surveillance
Case reporting is on a voluntary basis. Anderson Moores Veterinary Specialists is continuing to collate data and encourage colleagues to contact them regarding suspected, or confirmed, cases via medicine@andersonmoores.com
Summary
CRGV is a potentially life-threatening disease affecting dogs in the UK. Diagnosis and management of these cases can be extremely challenging, and vets are urged to familiarise themselves with the clinical signs of the disease and how to approach suspected cases.
- An urgent need exists for ongoing research to try to define the aetiology, thus the most appropriate way to manage dogs with CRGV. To this end, a national charity, called the Alabama Rot Research Fund (www.arrf.co.uk), was launched in 2016 with the aim of raising money for research to improve preventive measures, diagnostic testing and treatment.
Acknowledgements
The authors would like to thank Kerstin Erles DrMedVet, FRCPath, DipACVP, MRCVS for providing the histopathology images for this article and Carolina Urraca DVM, CertVDI, DipECVDI, MRCVS for assistance with the ultrasound images. The authors would also like to thank all of the vets who have submitted clinical records and samples for histopathology to Anderson Moores Veterinary Specialists, the owners of affected dogs, and the New Forest Dog Owners Group and the Alabama Rot Research Fund for raising awareness of CRGV and fund-raising ongoing CRGV research.