My recent trip to India comprised two weeks of intense sun, gorgeous beaches and delicious food that truly tested the constitution of my stomach. The majority of my time, however, was spent doing what I had gone all the way out there to do: surgery – lots and lots of surgery!
After 10 weeks of my clinical EMS was shut down by the pandemic, I had felt the desire to both travel and gain extra experience wherever possible, and so a surgical course based in Goa sounded like the perfect solution.
Spays for days
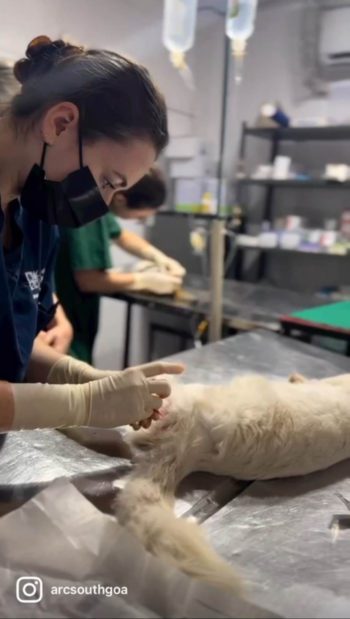
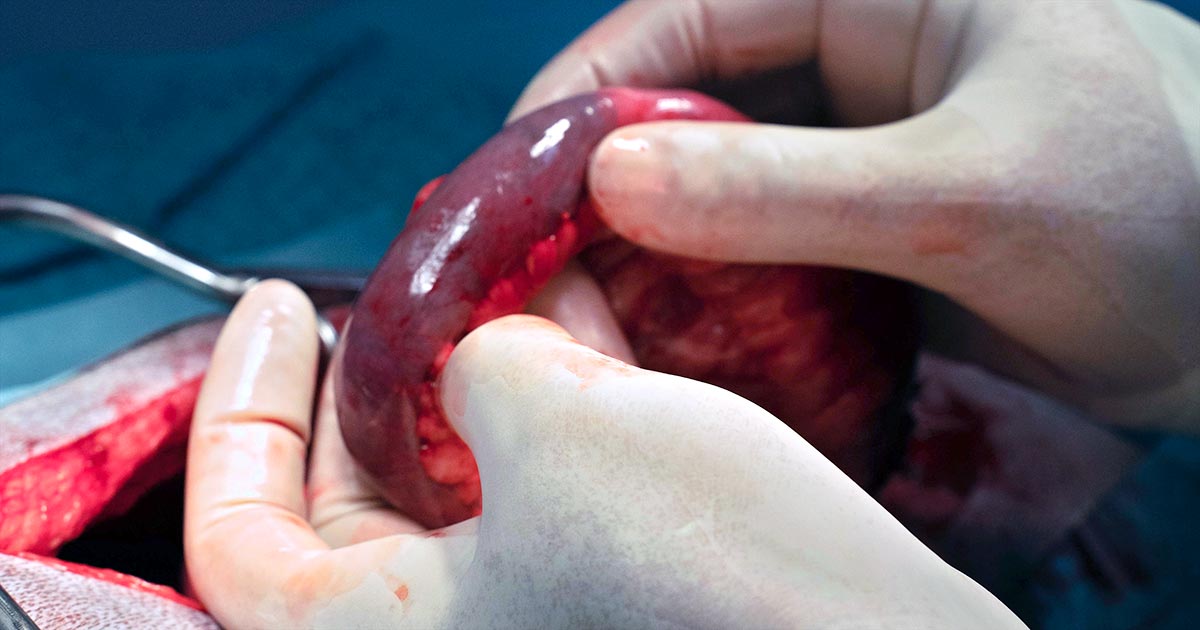
For my friend and I, the holy grail of all surgeries was the dreaded bitch spay. At the end of our placement – after what we presumed would initially be just watching, then maybe some helping, probably followed by a whole lot of cat castrating (the gateway surgery for newbies) – it was our hope that we would maybe (maayyybe) even be allowed to attempt a bitch spay for ourselves.
It didn’t ever occur to me that I would be executing my first bitch spay, from start to finish, by day three. Nor did I ever imagine that I would leave having done a total of 10… essentially one per day (although on some days we did two each).
Ironically, cat castrates were few and far between – even dog castrates for that matter – and of the 25 total surgeries I performed in those weeks, 16 were spays.
Left in the dark
The main thing I took away from the trip (aside from sore fingers) was a newfound appreciation for the fundamentals of surgery.
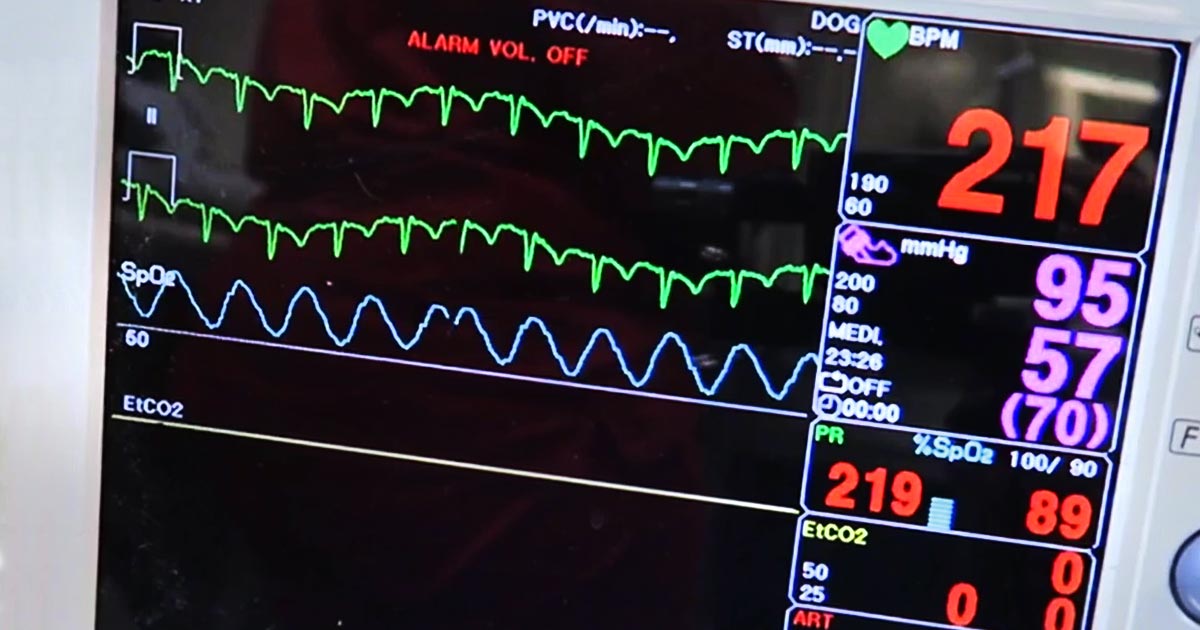
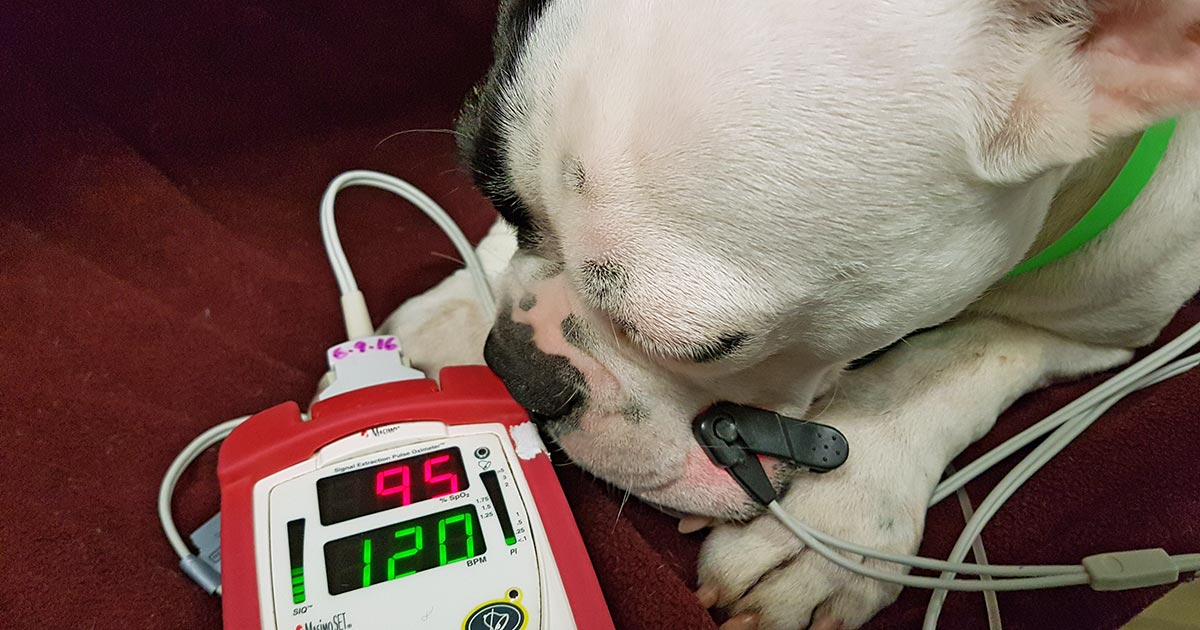
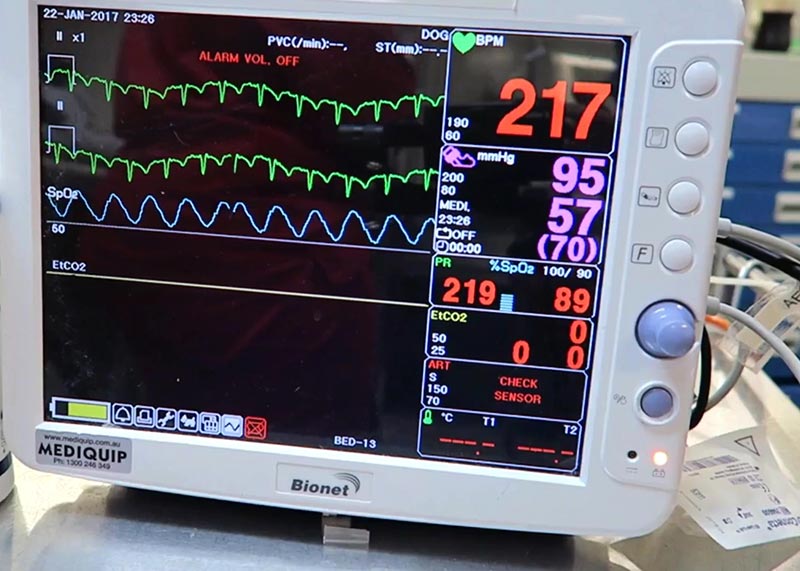
As was initially advertised to us, the clinic we found ourselves working in was charity based, and so lacked many of the facilities I think I’d learned (without even realising) to take for granted back home. Instruments were sterilised in an autoclave, there was no inhalant anaesthesia available, and no patient monitoring beyond CRT, pulse and breathing rate.
Plus, since there was no surgical lighting, and only one table was directly beneath the light, it meant a really deep-chested bitch spay on the other end of the room felt like operating in the dark.
How does it feel?
With no surgical lighting and only one head-torch to share between the two of us, the vet monitoring us joked towards the end of the placement that I could perform surgery by braille.
Although I wouldn’t recommend this approach to anyone, it gave me an incredible appreciation for the feel of normal versus abnormal anatomy – and that’s something no amount of revision or surgical observance could ever have given me.
Anaesthesia was purely parenteral, with top-ups being given as needed. We were all quite surprised by how well this worked for the majority of surgeries, with only a few hiccups along the way (and by hiccups I mean that, on one occasion, my patient turned around to look at me while I still had my fingers inside its abdomen).
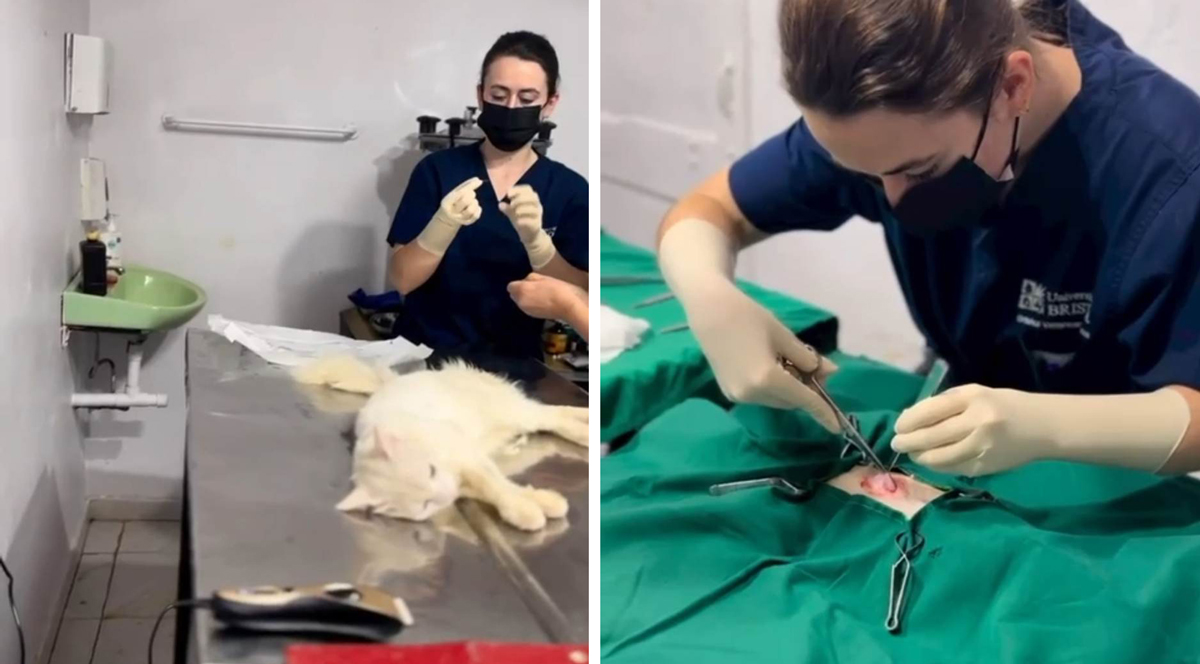
Expect the unexpected
No matter how much they teach you, or how well you learn the steps, there will always be a surgery – usually a bitch spay – that throws you a curveball (unfortunately, our patients haven’t read the textbook and are under no obligation to behave).
Whether it’s a ginormous blood vessel masquerading in a portion of facia, or a large glob of fat obscuring your view, every spay (even every castrate) has the potential to be entirely different to the previous one; surgery is not an endeavour for people who can’t roll with the punches or adapt their plan to a new situation.
I’ve heard the phrase, “no plan ever survives first contact with the enemy”, but I think my own proverb would read: “No surgical plan ever survives first contact with the patient.”
Well taught
One of the best instruments a vet can have at their disposal is support. My friend and I could not have asked for a better teacher, and the skills taught to us will undoubtedly be invaluable to us during the next stage of our careers.
I wouldn’t say that surgical programmes like this are for the faint hearted, but it provided me with experience that I simply would not have been able to gain had I not stepped out of my comfort zone.

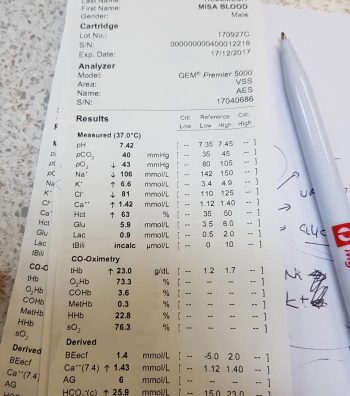



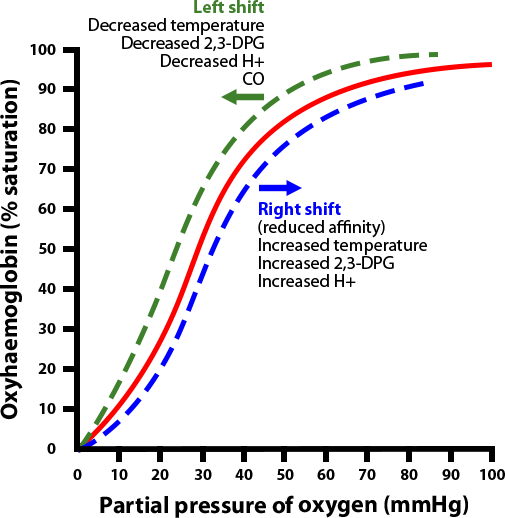 By the same token, a shift of the curve to the left means the Hgb has an increased affinity for oxygen, so is less likely to release it to the tissue. This normally occurs in the lungs.
By the same token, a shift of the curve to the left means the Hgb has an increased affinity for oxygen, so is less likely to release it to the tissue. This normally occurs in the lungs.
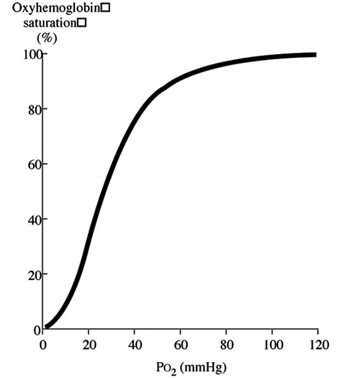 Initially, when the first oxygen molecule binds to Hgb’s, a change occurs in its conformation that increases its affinity for subsequent molecules of oxygen and, thus, hasten binding. This is depicted by the steep part of the OHDC curve.
Initially, when the first oxygen molecule binds to Hgb’s, a change occurs in its conformation that increases its affinity for subsequent molecules of oxygen and, thus, hasten binding. This is depicted by the steep part of the OHDC curve.