As discussed in part one of this blog series, a myriad of disease processes can lead to ionised hypocalcaemia (iHCa).
Despite this, only hypocalcaemia caused by eclampsia and hypoparathyroidism (primary or iatrogenic – post-surgical parathyroidectomy) are severe enough to demand immediate parenteral calcium administration.
Hypoparathyroidism is quite rare, so this blog will not explore the detailed pathophysiology behind this syndrome. However, it is worthwhile mentioning – aside from primary hypoparathyroidism – no other disease state requires long-term calcium supplementation.
Eclampsia, on the other hand, is the most common cause of clinical hypocalcaemia in dogs and cats. Multiple factors can predispose animals to the development of this phenomenon, so understanding the pathophysiology behind this potentially fatal disease will not only help with future diagnosis and treatment, but also help prevent this issue.
Periparturient occurrence
Eclampsia – also known as puerperal tetany or periparturient hypocalcaemia – occurs in the periparturient period anywhere from the final few weeks of gestation to four weeks postpartum, with the latter being the more common time frame of manifestation.
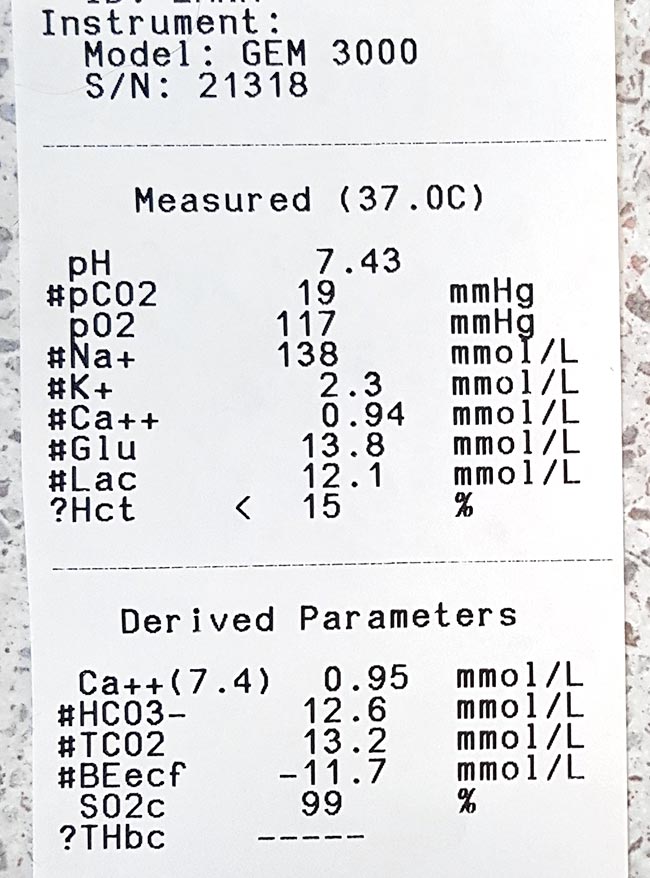
The serum concentration of ionised calcium (iCa) is often less than 0.9mmol/L in bitches or less than 0.8mmol/L in queens. It presents as muscle fasciculation and tetany, but not usually in seizure since most patients maintain consciousness. Exceptions occur when these patients are left untreated – these patients may develop refractory seizures, cerebral oedema and death.
The increased muscle activity generates a lot of heat and uses a significant amount of glucose; therefore, hyperthermia and hypoglycaemia are common sequelae in patients with delayed presentations.
Reduced iCa
Eclampsia occurs as a result of reduced iCa in the extracellular compartment. In lactation-associated hypocalcaemia, it is the result of the body’s inability to maintain serum iCa through increased osteolytic activity and gastrointestinal calcium absorption, and reduced renal calcium excretion to compensate for the loss of calcium through milk production.
Other factors often predispose animals to developing eclampsia. These can include poor periparturient nutrition, excessive calcium supplementation and large litter size.
Excessive calcium supplementation in the prenatal period causes parathyroid gland atrophy, preventing parathyroid hormone release – resulting in reduced gastrointestinal calcium absorption and osteoclastic activity, and increased kidney calcium loss.
Clinical signs
Clinical signs can progress rapidly and become fatal if left untreated.
In the early phases, non-specific signs can present as:
- facial pruritus
- hyperaesthesia
- panting
- tremors
- muscle fasciculations
- paresis
- ataxia
Within a few hours, these clinical signs rapidly progress to rigidity, and tonic and clonic spasms with opisthotonos. By this stage, animals will develop severe tachycardia, tachypnoea and hyperthermia. Without treatment, a high mortality rate exists.

Patients presenting with eclampsia require immediate medical intervention, as well as concurrent supportive therapy. The acute management of clinical iHCa is the same, regardless of the cause, and will be discussed in detail in part three.
Supportive therapies required to manage and prevent a patient relapsing in eclampsia often include active cooling and glucose supplementation. In cases that seizure, anti-seizure medications – such as diazepam and barbiturates – and mannitol for cerebral oedema may be required.
Prevention
Even before getting to the stage where an animal requires treatment, all effort must be taken to prevent a dam from developing hypocalcaemia. This can be easily achieved by improving the calcium content of the food during the perinatal period, as well as reducing the milk demand by early weaning kittens or puppies. This is likely particularly helpful for those with a history of eclampsia or with large litters.
From the second half of gestation, it is recommended a commercial formulation of puppy/kitten food (1% to 1.8% calcium and 0.8% to 1.6% phosphorus) is to be fed to the dam without any additional minerals or vitamin supplementation.
Postpartum calcium is similar to the second half of gestation, requiring a diet containing at least 1.4% calcium with a 1:1 ratio with phosphorus (most balanced growth formula for puppies and kittens).
Less demand
Early supplementation of puppies and kittens with commercial milk formula will significantly reduce the lactation demand on the dam. Together with this, starting at aged three to four weeks, solids can be introduced at this time. These techniques will be particularly helpful to those with a history of previous eclampsia or those with large litter sizes.
Aside from the parenteral calcium supplementation required, other supportive therapy – such as active cooling, IV fluid therapy and glucose supplementation – may be required.
Long term, the dam’s nutritional content of calcium must be optimal from the second half of gestation. All additional calcium or other vitamins and mineral supplementations should not occur prior to parturition.
In the postpartum dam with a history of eclampsia or that is at risk, changing to a nutritionally balanced commercial food aim for growing puppies and kittens is ideal. Early weaning – or abrupt weaning if hypocalcaemia is severe – may be required in severe cases or those with a high risk of relapse/development.





 To start us off, no real benefit exists in increasing a PCV above 30%, unless you are anticipating further losses. The reason being is oxygen delivery to tissues is optimised at that level and administering more does not add significant benefit.
To start us off, no real benefit exists in increasing a PCV above 30%, unless you are anticipating further losses. The reason being is oxygen delivery to tissues is optimised at that level and administering more does not add significant benefit.